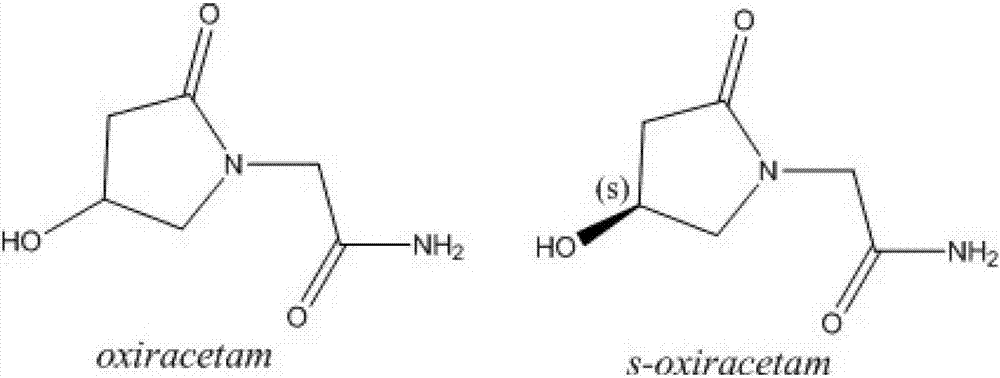
Levo-oxiracetam particle and preparation method thereof
A granule and prescription technology, applied in the field of levooxiracetam granules and the preparation thereof, can solve the problems of difficult particle size control, easy adhesion and blockage, strong hygroscopicity of granules, etc., and achieves a simple and feasible preparation process, and is not easy to absorb moisture. The effect of uniform particle size of lumps and particles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
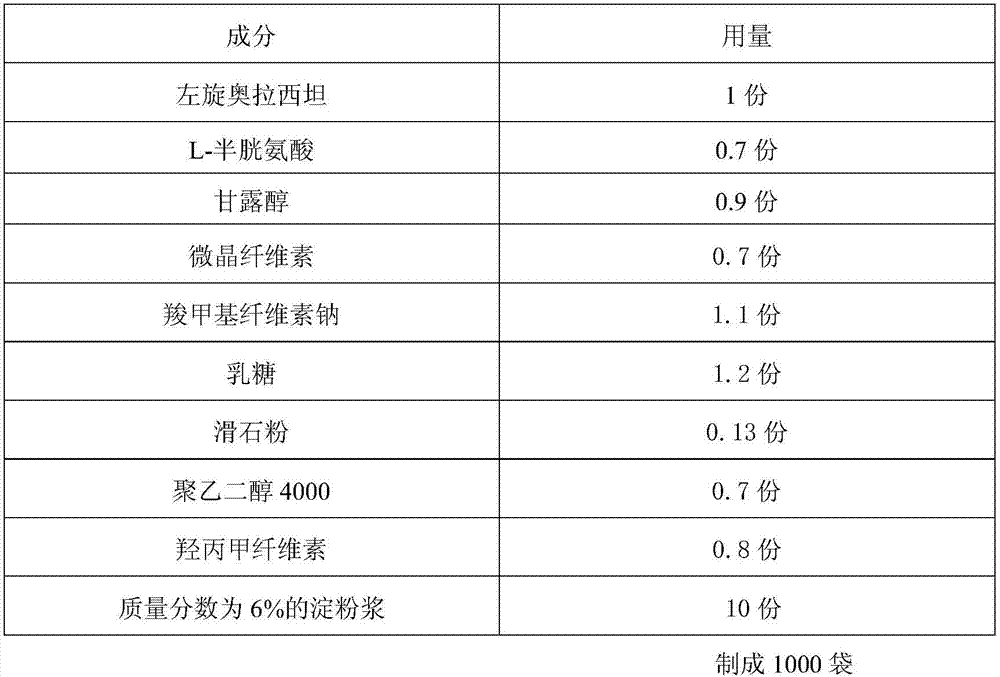
[0025] A levo-oxiracetam granule, prepared according to the following steps:
[0026]
[0027] Preparation process:
[0028] 1. Pretreatment of raw and auxiliary materials: Take the prescribed amount of levoxiracetam, L-cysteine, mannitol, microcrystalline cellulose, sodium carboxymethyl cellulose, and lactose in a universal grinder, and grind through 100 Mesh sieve, spare;
[0029] 2. Granulation: Take the mixed powder obtained after the pretreatment, place it in a wet granulator, add starch slurry with a mass fraction of 6%, start the granulator (install a 18-mesh nylon sieve), and start granulation;
[0030] 3. Drying: Put the wet granules into the fluidized bed, set the temperature of the hot bed at 50°C to 70°C, and start drying; observe the boiling and blasting conditions of the granules at any time to prevent the granules from sticking to the bottom of the pot, causing coking or gelatinization of the granules. The drying time is 50-55 minutes, and the moisture cont...
Embodiment 2
[0077] A levo-oxiracetam granule, prepared according to the following steps:
[0078]
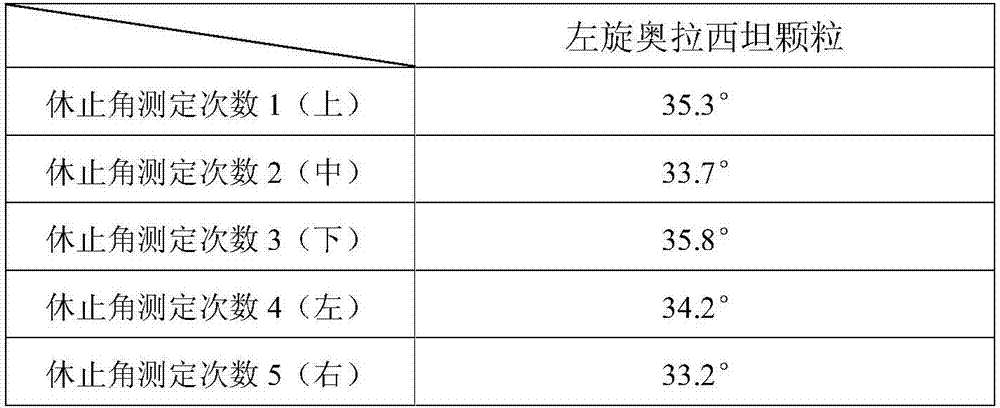
[0079] Preparation process: prepared according to the preparation process of Example 1. Carry out the test by the test method of embodiment 1, the Hughes angle test measurement result shows that this product granule fluidity is good, and the Hughes angle is lower than 37 °, and the loading difference test shows that the loading difference of this product is less than 5%, and the loading of this product Stable, controllable, the stability test results show that the quality of the samples in accelerated June is stable, and the quality of the long-term 24 months is stable, so the validity period of this product is at least 24 months. The test results of the influence of product prescription on the increase of impurities in the preparation process show that the impurities in the preparation process The increase is small, and the related substances only increase by 0.02% in the preparation pr...
Embodiment 3
[0081] A levo-oxiracetam granule, prepared according to the following steps:
[0082]
[0083] Preparation process: prepared according to the preparation process of Example 1. Carry out the test by the test method of embodiment 1, the Hughes angle test measurement result shows that this product granule fluidity is good, and the Hughes angle is lower than 35 °, and the loading difference test shows that the loading difference of this product is less than 4%, and the loading of this product Stable, controllable, the stability test results show that the quality of the samples in accelerated June is stable, and the quality of the long-term 24 months is stable, so the validity period of this product is at least 24 months. The test results of the influence of product prescription on the increase of impurities in the preparation process show that the impurities in the preparation process The increase is small, and the related substances only increase by 0.03% in the preparation pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| percent by volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com