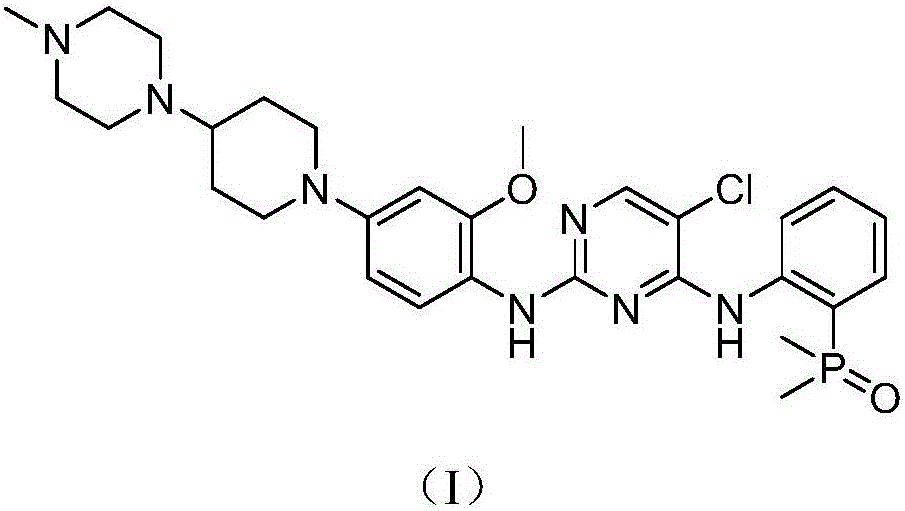
Homogeneous-phase 'one-pot' preparation method of Brigatinib key intermediate
An intermediate and key technology, applied in the field of homogeneous "one-pot" preparation, can solve the problems of incomplete reaction, low yield, cumbersome operation, etc., achieve high efficiency and yield, reduce solvent consumption, and shorten steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
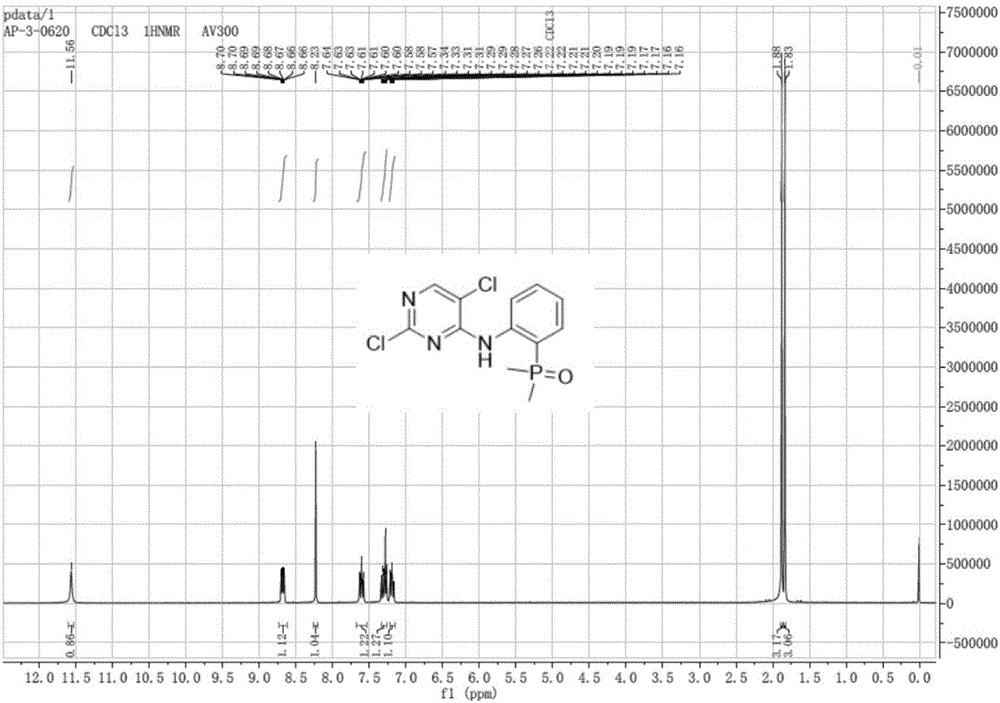
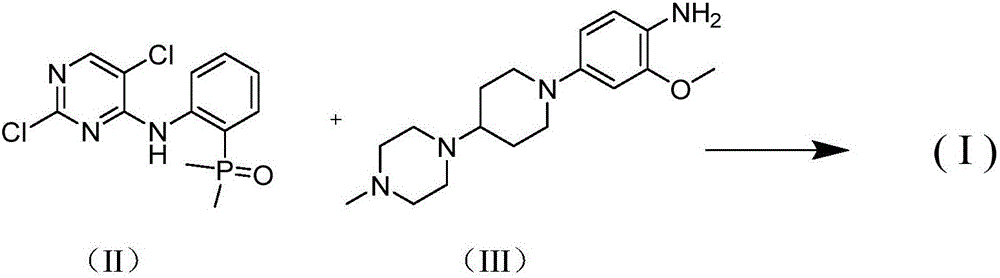
[0023] Add 2-iodoaniline (17.5g, 79.9mmol) and dimethylphosphine oxide (6.9g, 88.5mmol), palladium acetate (0.3g, 1.3mmol), Xantphos (0.77g, 1.3mmol), N,N-di Isopropylethylamine (22.7g, 175.8mmol), DMF (50mL), magnetic stirring. Under the protection of nitrogen, it was heated to 100°C for 6 hours, and the 2-iodoaniline was completely consumed as monitored by thin-layer chromatography. Cool to room temperature, add 2,4,5-trichloropyrimidine (17.5 g, 95.9 mmol), heat to 75 ° C for 6 hours, and monitor the completion of the reaction by thin-layer chromatography. Cool to room temperature, add 300 mL of water, adjust pH to 5 with 5% hydrochloric acid, extract with ethyl acetate (100 mL×3), wash with sodium bicarbonate solution (100 mL), wash with saturated sodium chloride solution (100 mL×2), anhydrous sulfuric acid Sodium dry. Suction filtration and concentration gave a crude brown solid, which was recrystallized from ethyl acetate / petroleum ether (volume ratio 1:2) to obtain 17...
Embodiment 2
[0025] Add 2-iodoaniline (8.6g, 39.3mmol) and dimethylphosphine oxide (3.4g, 43.6mmol), tetrakis (triphenylphosphine) palladium (0.92g, 0.8mmol), N,N-diisopropyl Ethylamine (11.2g, 86.7mmol), DMF (30mL), magnetic stirring. Under the protection of nitrogen, it was heated to 90°C for 8 hours, and the 2-iodoaniline was completely consumed as monitored by thin-layer chromatography. Cool to room temperature, add 2,4,5-trichloropyrimidine (8.6g, 47.0mmol), heat to 75°C for 6 hours, and monitor the completion of the reaction by thin-layer chromatography. Cool to room temperature, add 200 mL of water, adjust pH to 5 with 5% hydrochloric acid, extract with ethyl acetate (70 mL×3), wash with sodium bicarbonate solution (100 mL), wash with saturated sodium chloride solution (100 mL×2), anhydrous sulfuric acid Sodium dry. Suction filtration and concentration gave crude brown solid, which was recrystallized from ethyl acetate / petroleum ether (volume ratio 1:2) to obtain 6.8 g of off-whit...
Embodiment 3
[0027] Add 2-iodoaniline (7.8g, 35.6mmol) and dimethylphosphine oxide (3.1g, 39.7mmol), palladium acetate (0.13g, 0.58mmol), Xantphos (0.33g, 0.57mmol), N,N-di Isopropylethylamine (9.2g, 71.2mmol), DMF (30mL), magnetic stirring. Under the protection of nitrogen, it was heated to 100°C for 6 hours, and the 2-iodoaniline was completely consumed as monitored by thin-layer chromatography. Cool to room temperature, add 2,4,5-trichloropyrimidine (7.8g, 42.7mmol), heat to 75°C for 6 hours, and monitor the completion of the reaction by thin-layer chromatography. Cool to room temperature, add 200 mL of water, adjust pH to 5 with 5% HCl, extract with ethyl acetate (70 mL×3), wash with sodium bicarbonate solution (100 mL), wash with saturated sodium chloride solution (100 mL×2), anhydrous sulfuric acid Sodium dry. Suction filtration and concentration gave a crude brown solid, which was recrystallized from ethyl acetate / petroleum ether (volume ratio 1:2) to obtain 6.6 g of a near-white ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com