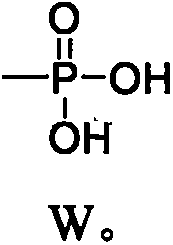Water-soluble tanshinone II A derivative, preparation and application thereof
A technology for tanshinone and derivatives, which is applied to water-soluble tanshinone IIA derivatives and the fields of preparation and application thereof, can solve the problems of poor water solubility, low blood drug concentration, low bioavailability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
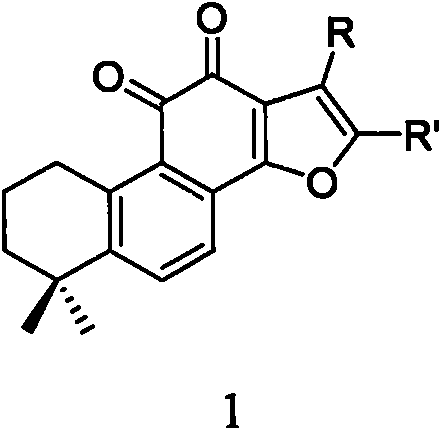
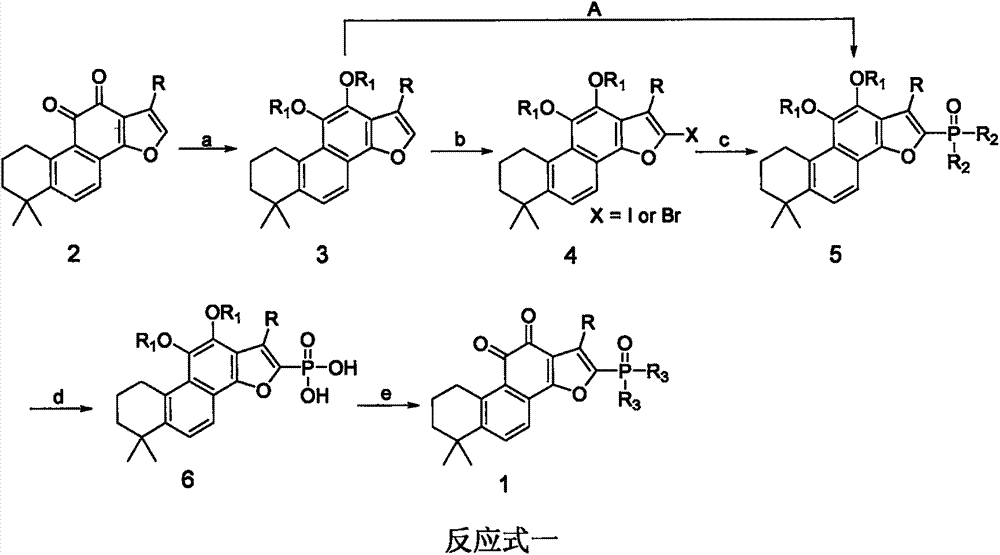
[0087] Embodiment one: the preparation of tanshinone IIA disodium phosphate:
[0088] (1) Synthesis of acetylated tanshinone IIA: Take tanshinone IIA (1.47g, 5mmol) and 10% palladium carbon (318mg, 3mmol) in 20mL dry tetrahydrofuran, replace it with hydrogen three times, and place it in a hydrogen atmosphere at room temperature (15-25°C) Stir until the red color fades to a colorless solution, inject acetic anhydride (2.1g, 20mmol) into the syringe, and then slowly inject pyridine (1.6g, 20mmol), dropwise in about 10 minutes, stir at room temperature 15-25°C for 3 hours, filter, 25 Concentrate under reduced pressure with a rotary evaporator with a vacuum of 0.1Mpa to remove the solvent, dilute with 50mL of ethyl acetate, wash with an equal volume of 1M dilute hydrochloric acid and water once each, and decompress the ethyl acetate solution at 25°C with a rotary evaporator with a vacuum of 0.1Mpa Concentrate to obtain a crude product, suspend the crude product in 30mL of methanol...
Embodiment 2
[0105] Example 2: Protective effect of tanshinone IIA disodium phosphate on focal cerebral ischemia-reperfusion injury in rats
[0106] (1) Purpose of the experiment
[0107] The model of focal cerebral ischemia-reperfusion injury in rats was established by means of suture middle cerebral artery occlusion (MCAO), and the prevention and treatment effect of tanshinone IIA disodium phosphate on focal cerebral ischemia-reperfusion injury in rats was observed.
[0108] (2) Experimental materials
[0109] 2.1 Test drugs
[0110] Name: Tanshinone IIA disodium phosphate (prepared in Example 1); traits: reddish-brown powder; solvent: 0.9% sodium chloride injection;
[0111] Test substance concentration: 3mg / ml
[0112] 2.2 Experimental control
[0113] Blank control (sham operation group): 0.9% sodium chloride injection;
[0114] Negative control (model group): 0.9% sodium chloride injection;
[0115] Positive control: Edaravone injection (must store); specification: 5ml: 10mg; ...
Embodiment 3
[0163] Example 3: Experimental Study on the Effect of Tanshinone IIA Disodium Phosphate on Myocardial Ischemia in Rats
[0164] (1) Purpose: To observe the effect of tanshinone IIA disodium phosphate on myocardial ischemia in rats
[0165] (2) Materials and methods
[0166] 2.1 Test drugs
[0167] Name: Tanshinone IIA Disodium Phosphate; Properties: Reddish-brown powder; Solvent: 0.9% Sodium Chloride Injection
[0168] Test substance concentration: 3mg / ml
[0169] 2.2 Experimental control
[0170] Blank control (sham operation group): 0.9% sodium chloride injection;
[0171] Negative control (model group): 0.9% sodium chloride injection;
[0172] 2.3 Animals
[0173] Strain: SD rat; Grade: SPF; Gender: male; Body weight: 220g
[0174] Source: Provided by Shanghai Sipro-Bikay Laboratory Animal Co., Ltd.
[0175] Total number of animals: 70
[0176] Animal production license number: SCXK (Shanghai) 2013-0016
[0177] Feeding conditions: room temperature 20-25°C, humidi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com