Preparation method of iron phosphate, iron phosphate prepared by method, preparation method of lithium iron phosphate, lithium iron phosphate prepared by method and lithium battery
A technology of lithium iron phosphate and iron phosphate, applied in the field of lithium batteries including lithium iron phosphate and lithium iron phosphate, can solve the problems of poor conductivity, low tap density, unstable performance, etc., and achieve improved filling and tap density. High, energy density enhancement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
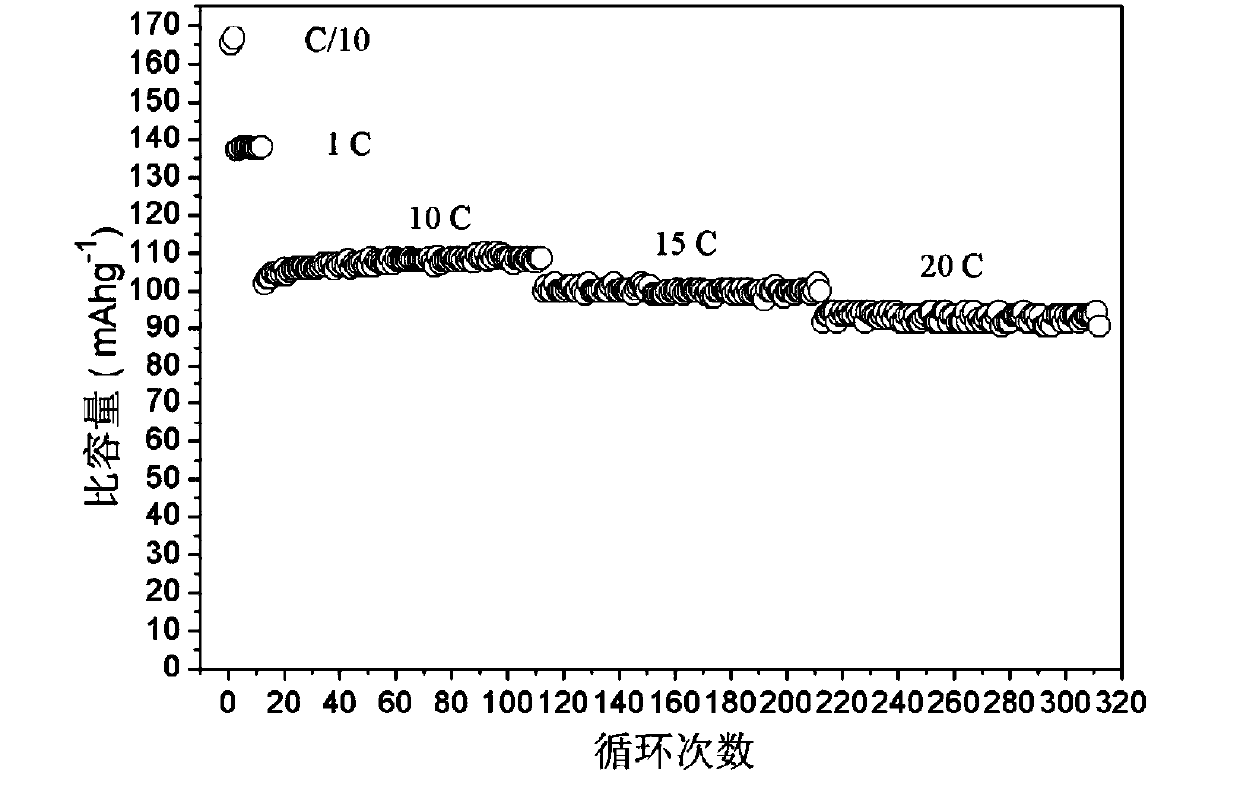
Image
Examples
preparation example Construction
[0050] A preparation method for an iron phosphate precursor prepared by lithium iron phosphate, said method comprising the following steps:
[0051] Steps to prepare ferrous sulfate : Add reduced iron powder accounting for 15% by weight of the aqueous solution to an aqueous solution containing 30% sulfuric acid and 1-10% organic acid, and control the temperature of the aqueous solution at 60-90°C to allow the reduced iron powder to react with the solution. The time is controlled within 5-10 hours. After the reaction is completed, the first reaction solution is obtained, and the first reaction solution is subjected to high magnetic filtration to remove iron element and high-priced iron ions, thereby obtaining an aqueous solution of ferrous sulfate;
[0052] The organic acid is at least one of lactic acid, malic acid, succinic acid, phytic acid or derivatives thereof (referring to derivatives of lactic acid, malic acid, succinic acid or phytic acid);
[0053] The steps of pre...
Embodiment 1
[0084] The preparation of nano-spherical ferric phosphate: put the iron with high-purity iron content greater than 99.8% into the reaction tank filled with 30% sulfuric acid and 1% phytic acid derivative (in mass fraction) as the lining of the acid solution, After heating to 95°C and fully reacting for 20 hours, the pH value is 3; filter, demagnetize, and remove impurities to obtain a pure ferrous sulfate solution with a concentration of 170g / L. Put 500L of the ferrous sulfate solution into the enamel oxidation kettle. At the same time, 72 kg of dissolved diammonium hydrogen phosphate and 50 kg of oxidant ammonium persulfate were added to the oxidation kettle within 10 minutes, and after full reaction, pressure filtration, demagnetization, cleaning, and impurity removal were obtained to obtain initially pure nano-iron phosphate slurry. The value is 5.
[0085] Use phosphoric acid to adjust the acidic pH value of the initial slurry to 2.5, and fully disperse it, put it into the...
Embodiment 2
[0089] Preparation of nano-spherical ferric phosphate: Put iron with a high-purity iron content greater than 99.8% into a reaction tank filled with 30% sulfuric acid and 5% phytic acid derivatives, heat to 90°C, and fully react for 30 hours. The pH value is 3; filter, Demagnetize and remove impurities to obtain ferrous sulfate solution. Put 400L of ferrous salt solution into the enamel oxidation kettle. At the same time, add 58kg of dissolved diammonium hydrogen phosphate and 58kg of ammonium persulfate into the oxidation kettle within 15 minutes. After fully reacting Press filtration to clean and remove impurities to obtain initially pure nano-iron phosphate slurry.
[0090] Use phosphoric acid to adjust the acidic pH value of the initial slurry to 2.8, add a nanometer control agent to 1%, and fully disperse it, put it into the reaction kettle, heat it to 95°C, and make the initial ferric phosphate form nanocrystals, filter and rinse, and vacuum at 110°C Dry to obtain nano-sp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com