Biphenyl-type magnetic liquid crystal monomer substituted by alkylimidazole tetrahalide ferric salt and preparation method thereof
A technology of alkyl imidazoles and liquid crystal monomers is applied in the field of ring-opening metathesis polymerization to synthesize a class of structures, which can solve problems such as unreported, and achieve good substrate universality, controllable structure and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
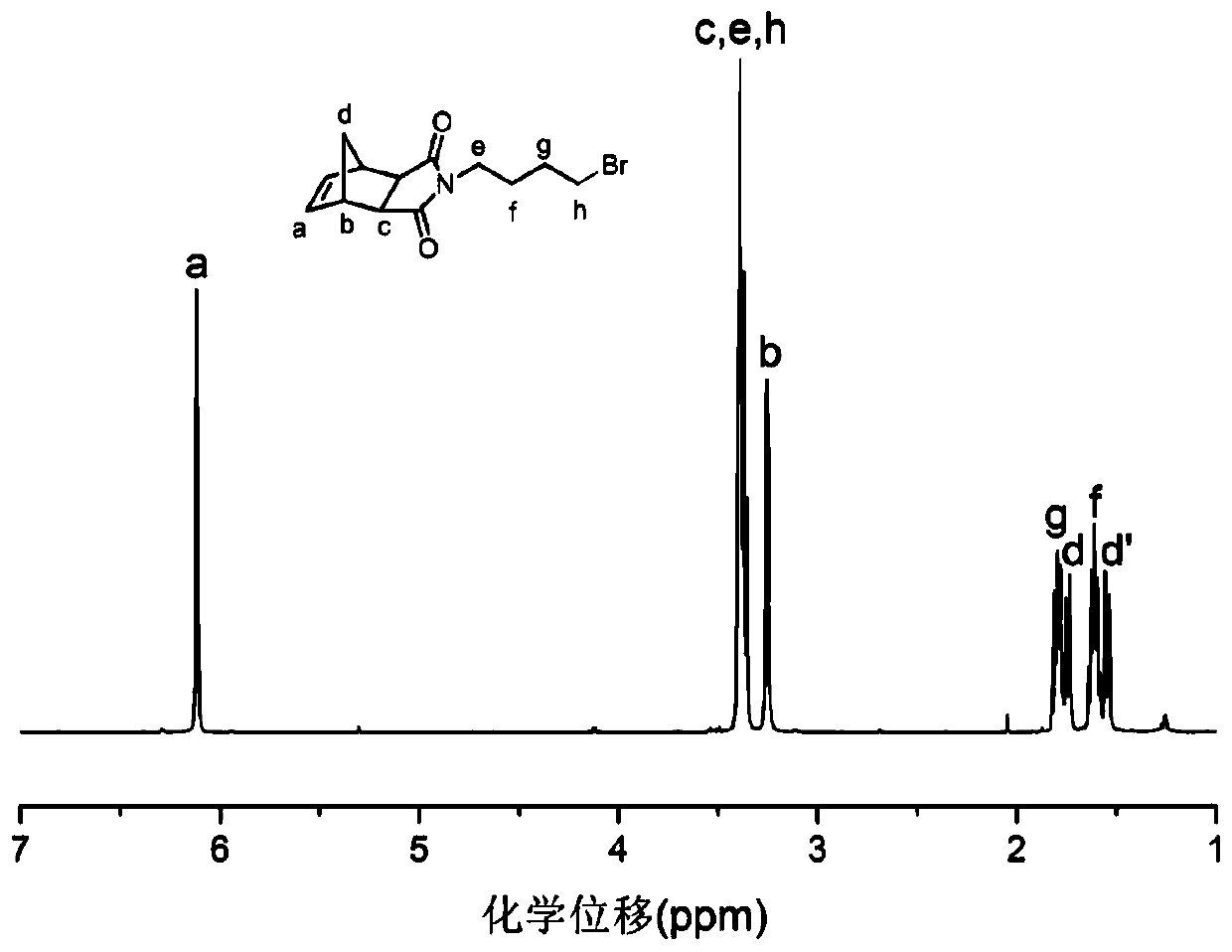
[0067] The synthesis of intermediate product a, its chemical structure is as follows:
[0068]
[0069] (1) Add 1,4-dibromobutane (13.2190g, 60mmol) and anhydrous potassium carbonate into a 250mL round-bottomed flask, and stir with a polytetrafluoroethylene magnet. 5-norbornene-2,3-dicarboximide (1.6651 g, 10 mmol) was added into 100 mL DMF to form solution A, and solution A was added dropwise into 1,4-dibromobutane. After the addition was completed, the stirring reaction was continued for 48h to make the reaction complete.
[0070] (2) After the reaction, the resulting mixed system was filtered, the filtrate was transferred to a 500mL separatory funnel, 150mL distilled water was added, and then extracted three times with 50mL dichloromethane, the dichloromethane phases were combined, and anhydrous magnesium sulfate was added to dry overnight. The dried liquid was filtered, the filtrate was collected, and the solvent was removed on a rotary evaporator to obtain a crude pro...
Embodiment 2
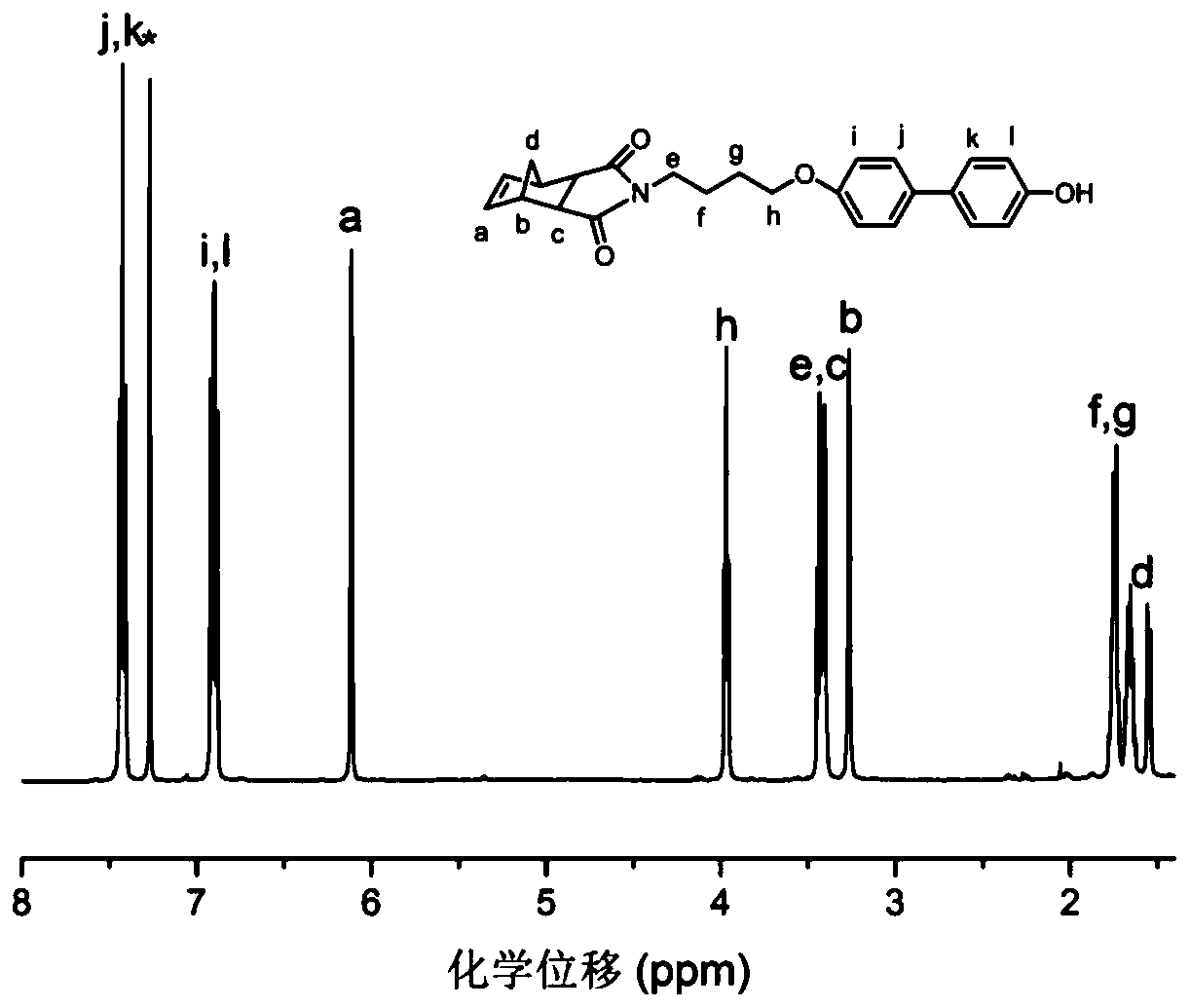
[0073] The synthesis of intermediate product b, its chemical structure is shown below:
[0074]
[0075] (1) Add phenol (4.7023g, 25mmol) and potassium hydroxide (6.9803g, 50mmol) into a 250mL double-necked bottle, add a polytetrafluoroethylene magnet, then add 50mL of acetone to the bottle, and heat to 80°C, react for 30 minutes. Add 30 mL of acetone to intermediate product a (1.4909 g, 5 mmol) to form solution B, and drop solution B into the reaction flask. After the dropwise addition is completed, continue to stir and react at 80°C for 20h to make the reaction complete.
[0076] (2) After the reaction, the resulting mixture was transferred to a 500 mL separatory funnel, 100 mL of distilled water was added, and fully acidified with hydrochloric acid, then extracted three times with 50 mL of ethyl acetate, the ethyl acetate phase was combined, and anhydrous magnesium sulfate was added to dry overnight. The dried liquid was filtered, the filtrate was collected, and the so...
Embodiment 3
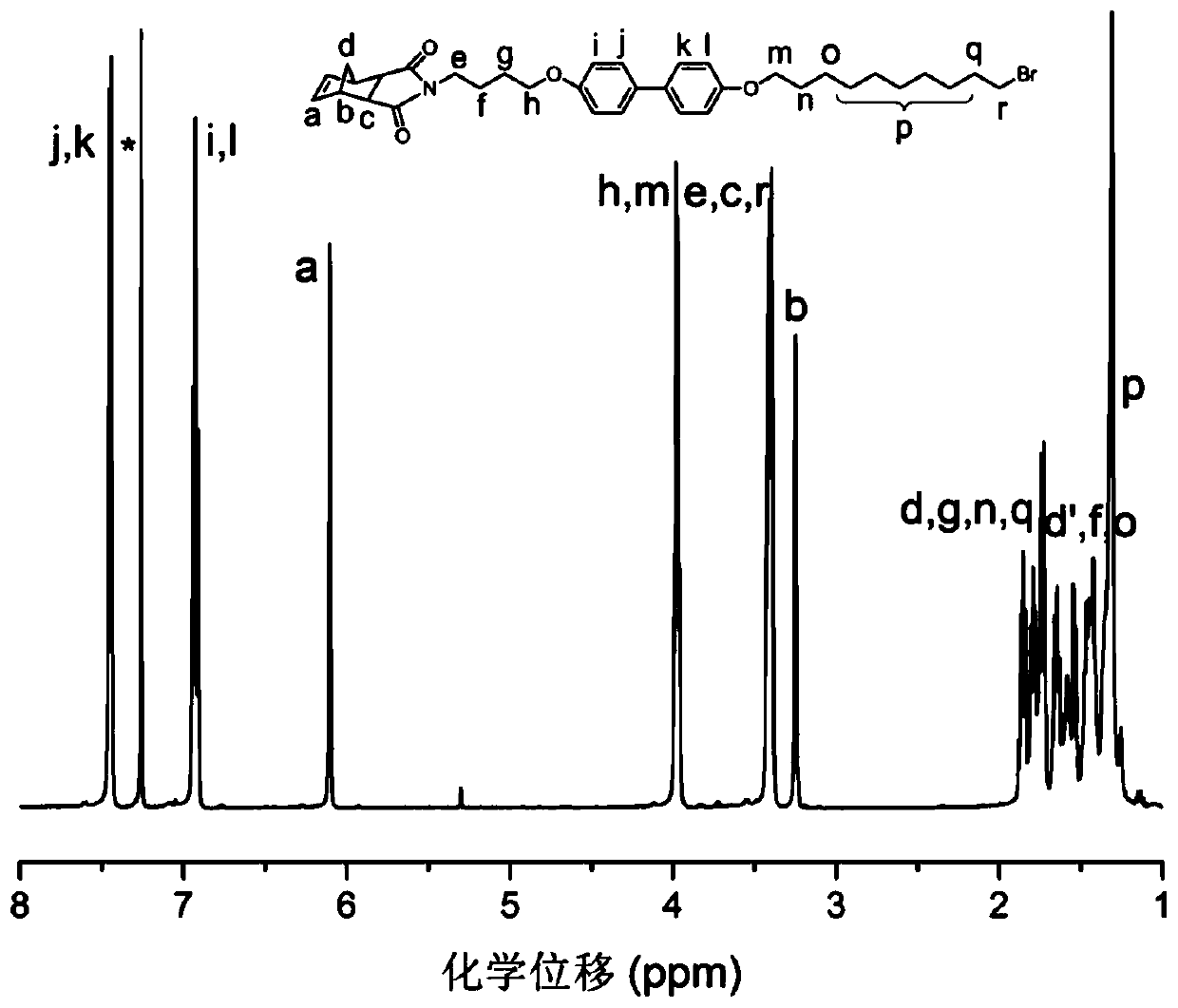
[0079] The synthesis of intermediate product c, its chemical structure is shown below:
[0080]
[0081] (1) Add intermediate product b (403.47mg, 1mmol), 1,10-dibromodecane (6.062g, 20mmol), potassium hydroxide (57mg, 1mmol) into a 100mL round bottom flask, and add polytetrafluoroethylene Vinyl fluoride magnet, then add 20mL acetone to the bottle, heat to 60°C, and react for 20h.
[0082](2) After the reaction, the resulting mixture was transferred to a 125 mL separatory funnel, 35 mL of distilled water was added, and extracted three times with 50 mL of ethyl acetate, the ethyl acetate phases were combined, and anhydrous magnesium sulfate was added to dry overnight. The dried liquid was filtered, the filtrate was collected, and the solvent was removed on a rotary evaporator to obtain a crude product.
[0083] (3) The crude product was separated by column chromatography, petroleum ether / ethyl acetate was used as eluent, the product was collected, the solvent was removed by...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com