A novel reversed-phase/weak cation-exchange mixed-mode chromatographic stationary phase and its preparation method
A mixed-mode chromatography and weak cation exchange technology, applied in the field of chromatographic separation research, to achieve the effects of easy-to-obtain reaction raw materials, mild reaction conditions, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1, preparation of reversed phase / weak cation exchange mixed mode chromatographic stationary phase
[0053] (1) Take 30g spherical silica gel (particle size is 3μm, pore size is ), dispersed in 5mL / g hydrochloric acid solution (20wt%), ultrasonicated for half an hour, and shaken overnight on a rotary mixer. The silica gel was washed with deionized water until neutral, dried overnight at 110°C, and placed in a desiccator for later use.
[0054] (2) Disperse 15 g of pretreated silica gel microsphere matrix in 45 mL of dry toluene, add 15 mL of vinyltrichlorosilane, and react in a water bath at 40 °C at a magnetic stirring speed of 100 rpm for 24 h under nitrogen protection. After the reaction, wash with toluene, methanol and dichloromethane respectively, and dry at 80°C for 6h.
[0055] (3) Disperse the reacted silicon spheres in 100mL of methanol:water (V:V=1:2), add 15g of cysteine and 0.15g of azobisisobutyronitrile, and magnetically Stir the reaction for ...
Embodiment 2
[0062] Embodiment 2, separation polycyclic aromatic hydrocarbon mixture
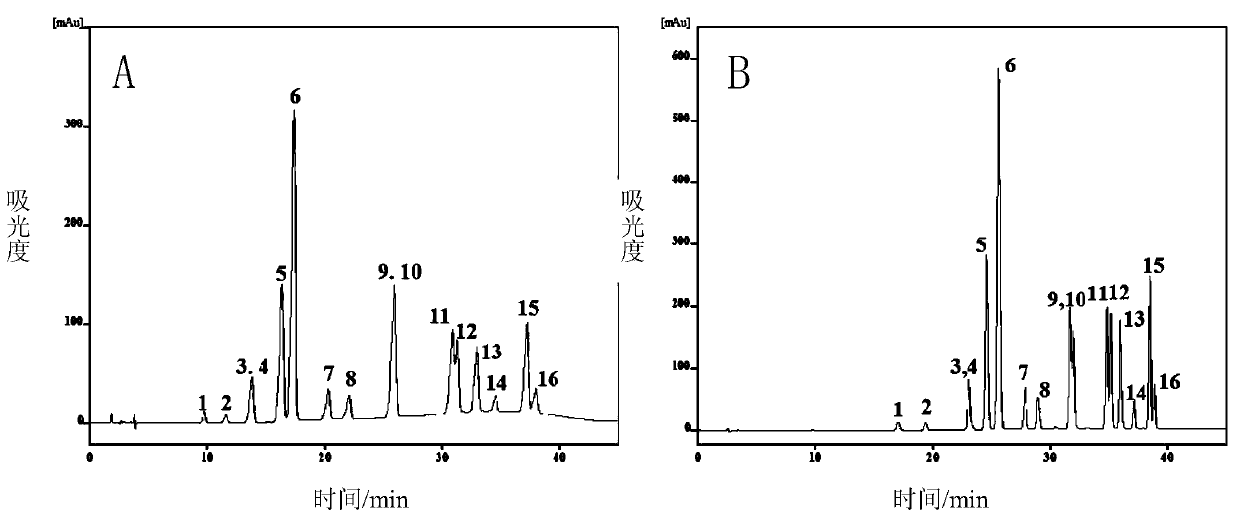
[0063] Using the reverse phase / weak cation exchange mixed mode chromatographic stationary phase prepared in Example 1, the stationary phase was packed into a 4.6×150mm stainless steel chromatographic column by the high-pressure homogenization method, and 16 kinds of polycyclic aromatic hydrocarbon mixtures were carried out in the reverse phase mode. separate. As a comparison, under the same chromatographic conditions, 16 polycyclic aromatic hydrocarbon mixtures were separated using a Shiseido reverse-phase chromatographic column (model: CAPCELL PAKC18MGII S3).
[0064] Chromatographic conditions: the sample concentration is 10 μg / mL, the sample loading volume is 10 μL, and the flow rate is 0.5 mL / min. Phase A: water; phase B: acetonitrile; gradient: 0-5min 60%-100%B, 5-45min 100%B. The detection wavelength is 254nm.
[0065] The separation chromatogram is as image 3 As shown (peak 1 represents napht...
Embodiment 3
[0066] Embodiment 3, separation polypeptide mixture
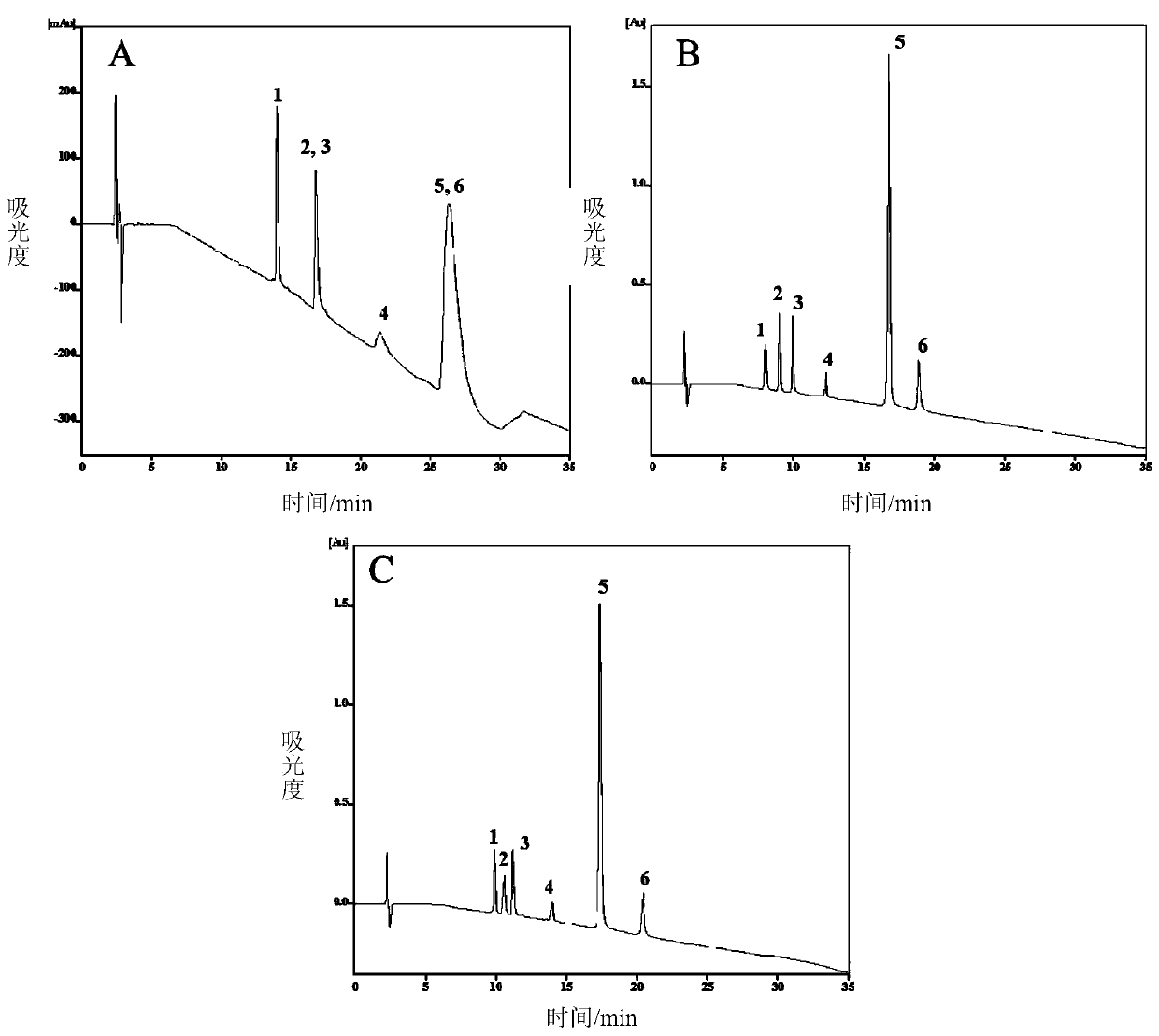
[0067] Using the reverse phase / weak cation exchange mixed-mode chromatographic stationary phase prepared in Example 1, the stationary phase was loaded into a 4.6×150mm stainless steel chromatographic column by the high-pressure homogenization method, and the mixture of 6 polypeptides was separated under different pH conditions .
[0068] The chromatographic conditions are: the sample concentration is 0.5 μg / μL, the sample loading volume is 20 μL, and the flow rate is 0.5 mL / min. Phase A: 98% water (20mM ammonium formate, pH 4.5, 6.0, 7.5) ~ 2% acetonitrile; phase B: 98% acetonitrile ~ 2% water; gradient: 0 ~ 30min2% ~ 95% B. The detection wavelength is 214nm.
[0069] The separation chromatogram is as Figure 4 , when the pH is low, the reversed-phase / weak cation exchange mixed-mode chromatographic stationary phase behaves as a reversed-phase separation mechanism, and cannot completely separate the six polypeptides; when...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com