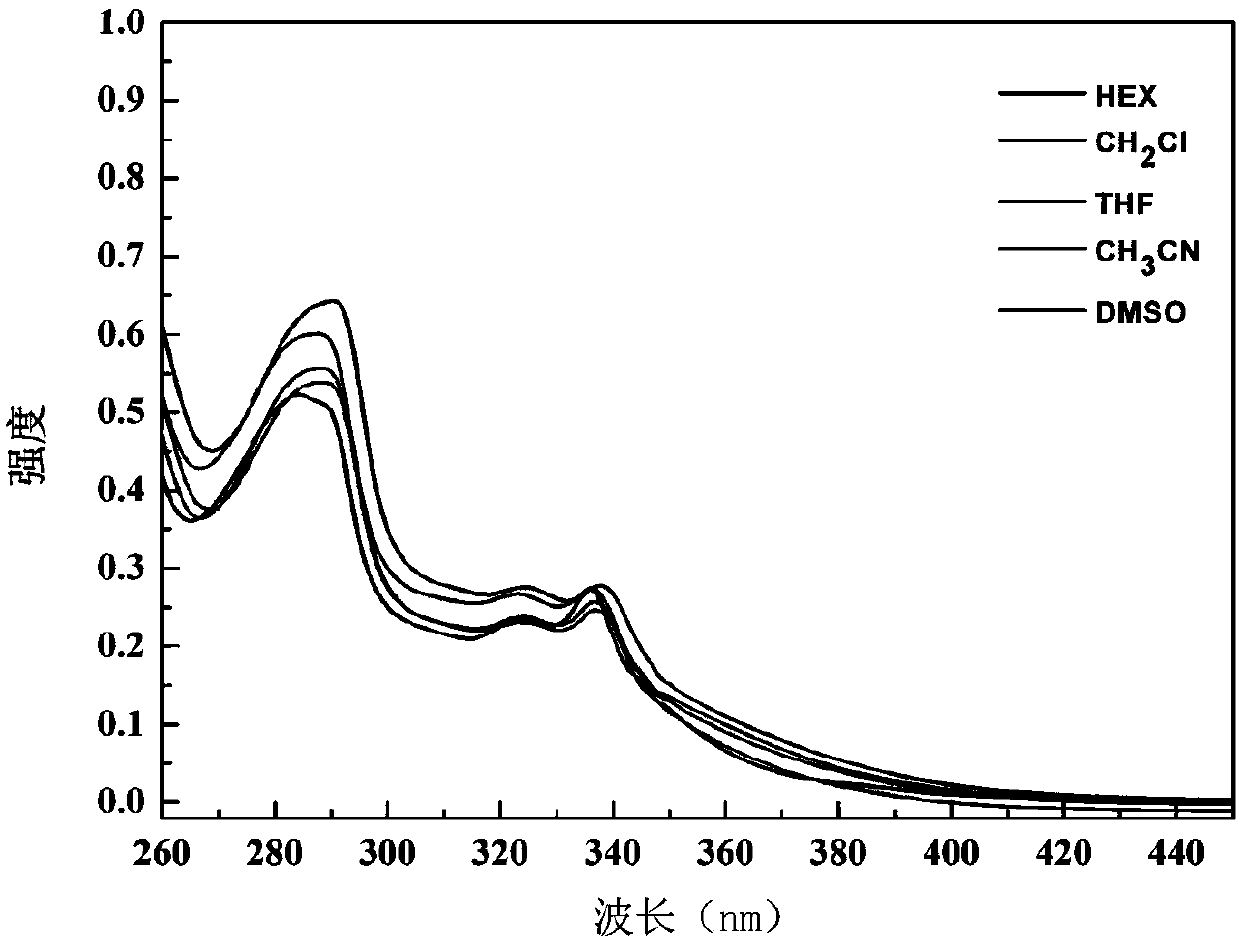
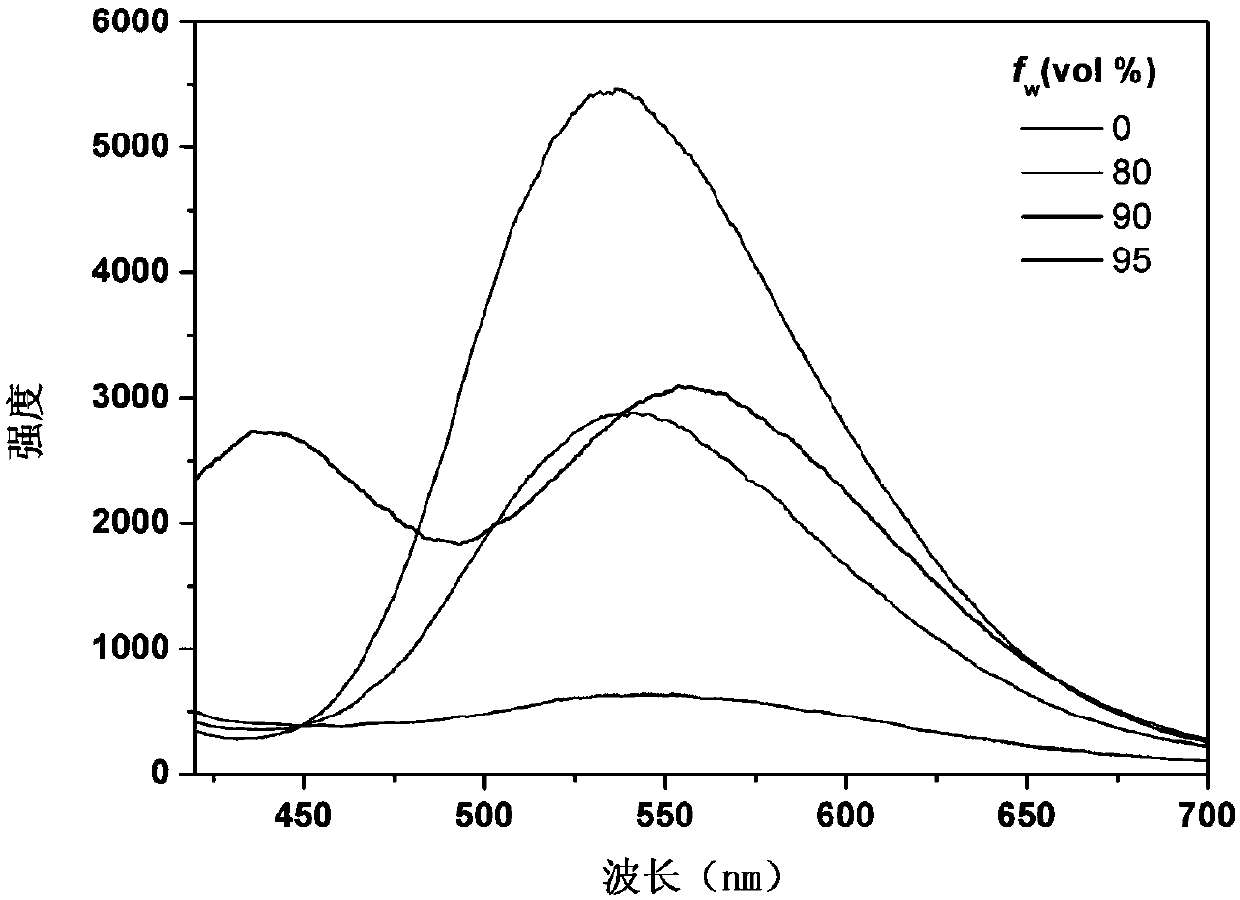
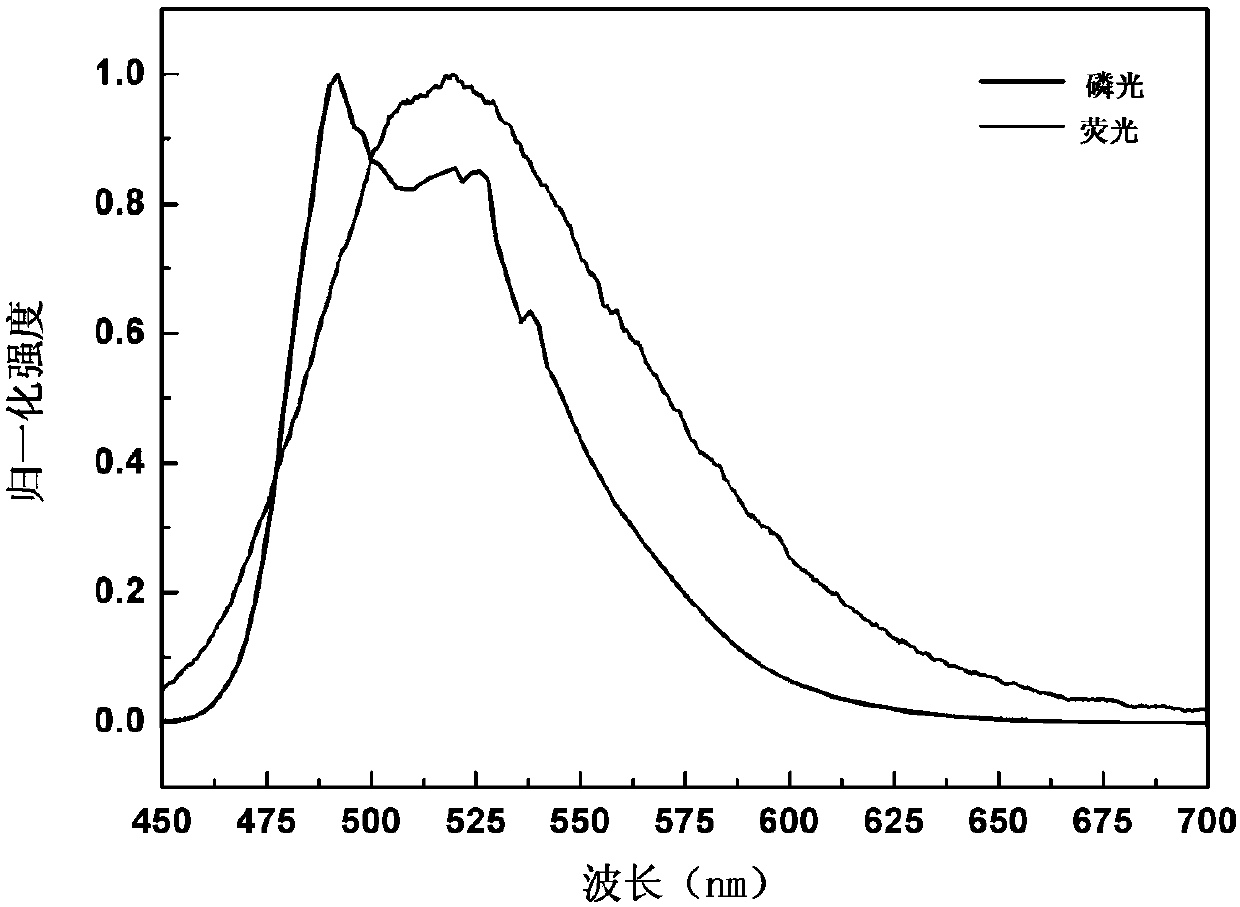
Compound comprising 9,9-dimethyl-9.10-dihydracridine and preparation and application thereof
A technology of dihydroacridine and dimethyl, which is applied in the application field of organic electroluminescent materials, can solve problems hindering development and practical application, and achieve easy operation, good thermal stability, high product yield and purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1. Preparation of (3,5-biscarbazole-9-phenyl)-(4-(9,9-dimethyl-9-acridine-10-)-phenyl)-methanone, steps as follows:
[0032](1) Synthesis and Characterization of 9,9'-(5-bromo-1,3-phenylene)bis(9H-carbazole)
[0033] Carbazole (8.35g, 50mmol), 1,3,5-tribromobenzene (7.85g, 25mmol), CuI (0.48g, 2.5mmol), 18-crown-6 (1.0g, 3.8mmol), carbonic acid Potassium (18.3 g, 75 mmol) was dissolved in o-dichlorobenzene (100 mL), heated to 180° C. and stirred for 24 h under nitrogen. After cooling to room temperature, saturated (NH 4 ) 2 CO 3 The solution was quenched, and the mixture was extracted with chloroform; the organic layers were combined, washed three times with distilled water, and dried over anhydrous magnesium sulfate. Then the solvent was spin-dried to obtain the crude product, which was separated by column chromatography to obtain the white solid compound 9,9'-(5-bromo-1,3-phenylene)bis(9H-carbazole) (6.0 g, yield 50 %). 1 H NMR (600MHz, CDCl 3 ): δ8.14...
Embodiment 2
[0040] Embodiment 2. Structure, preparation and performance of organic electroluminescent devices:
[0041] We have prepared a non-doped organic electroluminescence device, the structure of the device is: ITO / HATCN, thickness 20nm / TAPC, thickness 30nm / the said 9,9-dimethyl-9,10-dihydro The compound with acridine structure has a thickness of 25nm / TmPyPB, a thickness of 40nm / LiF, a thickness of 1nm / Al, and a thickness of 150nm; the structures of three doped organic electroluminescent devices are: ITO / HATCN, a thickness of 20nm / TAPC, a thickness of 30nm / all The compound containing 9,9-dimethyl-9,10-dihydroacridine structure (mass fraction 6%, 10%, 20%): CBP, thickness 25nm / TmPyPB, thickness 40nm / LiF, thickness 1nm / Al, thickness 150nm. Among them, HATCN is used as a hole injection layer, and TAPC is used as a hole transport layer; (3,5-biscarbazole-9-phenyl)-(4-(9,9-dimethyl-9-acridine-10- )-phenyl)-methanone (compound DCPDAPM) as the light emitting layer; TmPyPB as the electro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com