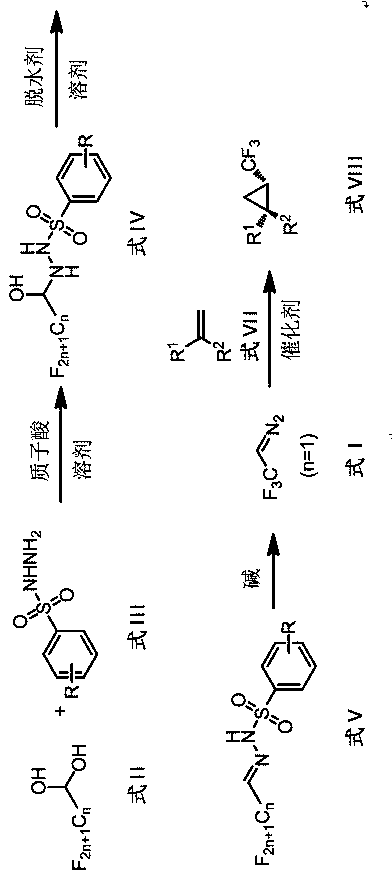
A kind of preparation method and application of perfluoroalkyldiazomethane
A technology of perfluoroalkyl and diazomethane, which is applied in the preparation of halogenated hydrocarbons, the preparation of sulfonic acid amides, chemical instruments and methods, etc., can solve the problems of cumbersome operation, explosion danger, inapplicability, etc. Wide substrate range and well tolerated effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 3
[0049] The preparation of embodiment 1 trifluoroacetaldehyde benzenesulfonylhydrazone 4a
[0050] The reaction formula of steps (1)~(2) is as follows:
[0051] ;
[0052] (1) Under nitrogen, add o-nitrobenzenesulfonylhydrazide 2a (10.9g, 50mmol) and 200mL ethyl acetate into a 250mL reaction flask, stir until dissolved, cool down to 0°C in an ice-water bath, add 10 drops of concentrated Sulfuric acid, add trifluoroacetaldehyde hydrate 1a (8.7g, 75mmol), react at 0°C until TLC monitoring shows that o-nitrobenzenesulfonyl hydrazide 2a disappears; add 50mL of 10% saline solution, separate the liquid, and wash the organic phase with saturated Washed twice with aqueous sodium chloride solution, dried over anhydrous magnesium sulfate, suction filtered, and the filtrate was dropped into 1000mL of n-hexane, a white solid was gradually precipitated, filtered, and dried in vacuo to obtain a white solid 3a (15.1g, yield 96%), without Purification, the next reaction directly.
[0053...
Embodiment 2
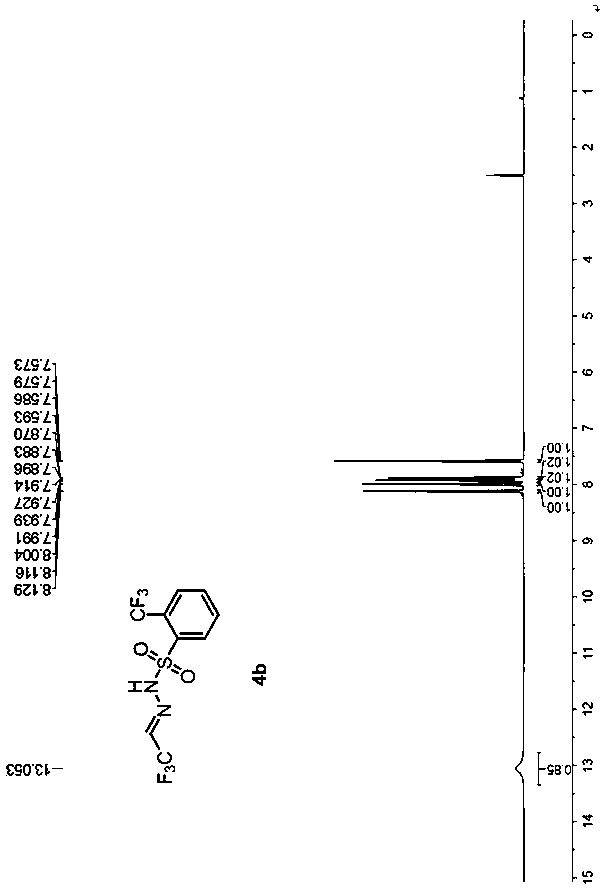
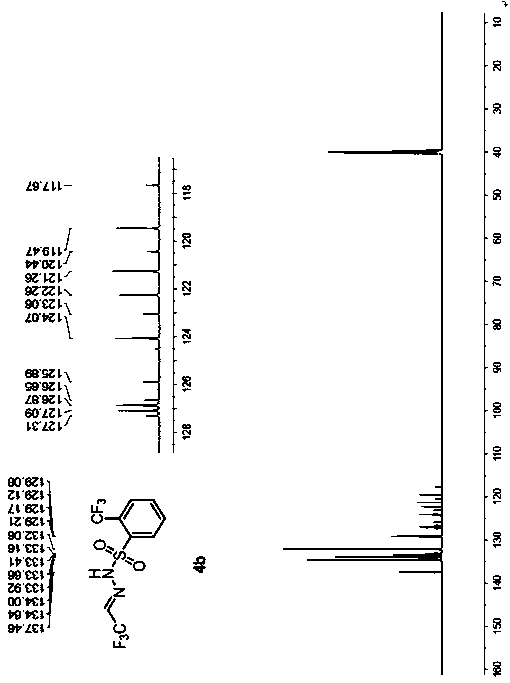
[0055] Example 2 Preparation of trifluoroacetaldehyde benzenesulfonylhydrazone 4b
[0056] The reaction formula of steps (1)~(2) is as follows:
[0057]
[0058] (1) Under nitrogen, add o-trifluoromethylbenzenesulfonyl hydrazide 2b (4.8 g, 20 mmol) and 40 mL of 1,2-dichloroethane into a 250 mL reaction flask, stir until dissolved, and cool to 0°C, add 10 drops of glacial acetic acid dropwise, add trifluoroacetaldehyde hydrate 1a (3.5g, 30mmol), react at 0°C until o-trifluoromethylbenzenesulfonyl hydrazide 2b disappears, add 10% saline 25mL, separated, the organic phase was washed twice with saturated aqueous sodium chloride solution, dried over anhydrous magnesium sulfate, filtered with suction, dropped into 300mL of n-hexane, a white solid gradually precipitated, filtered, and vacuum-dried to obtain a white solid 3b (15.1g , yield 96%), without purification, directly to the next step reaction.
[0059] (2) Under the condition of nitrogen protection, add 3b (3.15g, 10mmol...
Embodiment 3
[0061] Example 3 Preparation of pentafluoropropionaldehyde benzenesulfonylhydrazone 4c
[0062] The reaction formula of steps (1)~(2) is as follows:
[0063]
[0064] (1) Under nitrogen, add o-trifluoromethylbenzenesulfonyl hydrazide 2b (4.8 g, 20 mmol) and 40 mL of 1,2-dichloroethane into a 250 mL reaction flask, stir until dissolved, and cool down in an ice-water bath to 0°C, add 10 drops of glacial acetic acid dropwise, add pentafluoropropionaldehyde hydrate 1b (5.0 g, 30 mmol), react at 0°C until o-trifluoromethylbenzenesulfonyl hydrazide 2b disappears, add 10% 25 mL of common saline solution, separated, the organic phase was washed twice with saturated aqueous sodium chloride solution, dried over anhydrous magnesium sulfate, filtered with suction, the filtrate was dropped into 200 mL of n-hexane, a white solid gradually precipitated, filtered, and vacuum-dried to obtain a white Color solid 3c (7.0 g, yield 90%) was directly used for the next step without purification....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com