PEG-modified medicinal kininogenase and preparation method and application thereof
A kininogenase and reaction technology, applied in the field of polyethylene glycol modification, can solve the problems of repeated administration, short half-life, redness and swelling at the injection site, and achieve high biological activity and efficacy, reduced immunogenicity, The effect of half-life extension
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Example 1 Separation and purification of porcine pancreatic kininogenase single components KLK1b, KLK1a and KLK1c
preparation example 1
[0082] Porcine pancreatic kininogenase (from Changzhou Qianhong Biochemical Pharmaceutical Co., Ltd.) was diluted to 6 mg / mL with liquid A, and purified by ion-exchange chromatography. Purified chromatography conditions: ion exchange medium (QFF), A solution: 50mM Tris-HCl (pH9.0), B solution: 50mM Tris-HCl (pH9.0) containing 1M NaCl; flow rate 10mL / min, The detection wavelength is 280nm.
[0083] Loading: the above dilution of porcine pancreatic kininogenase was bound to a QFF ion exchange column.
[0084] Equilibration: Flush 5 column volumes with solution A.
[0085] Elution: The mobile phase ratio is 12% B, 88% A, after elution of 10 column volumes, gradient elution is performed. Gradient conditions were 12% B to 30% B, 30 column volumes.
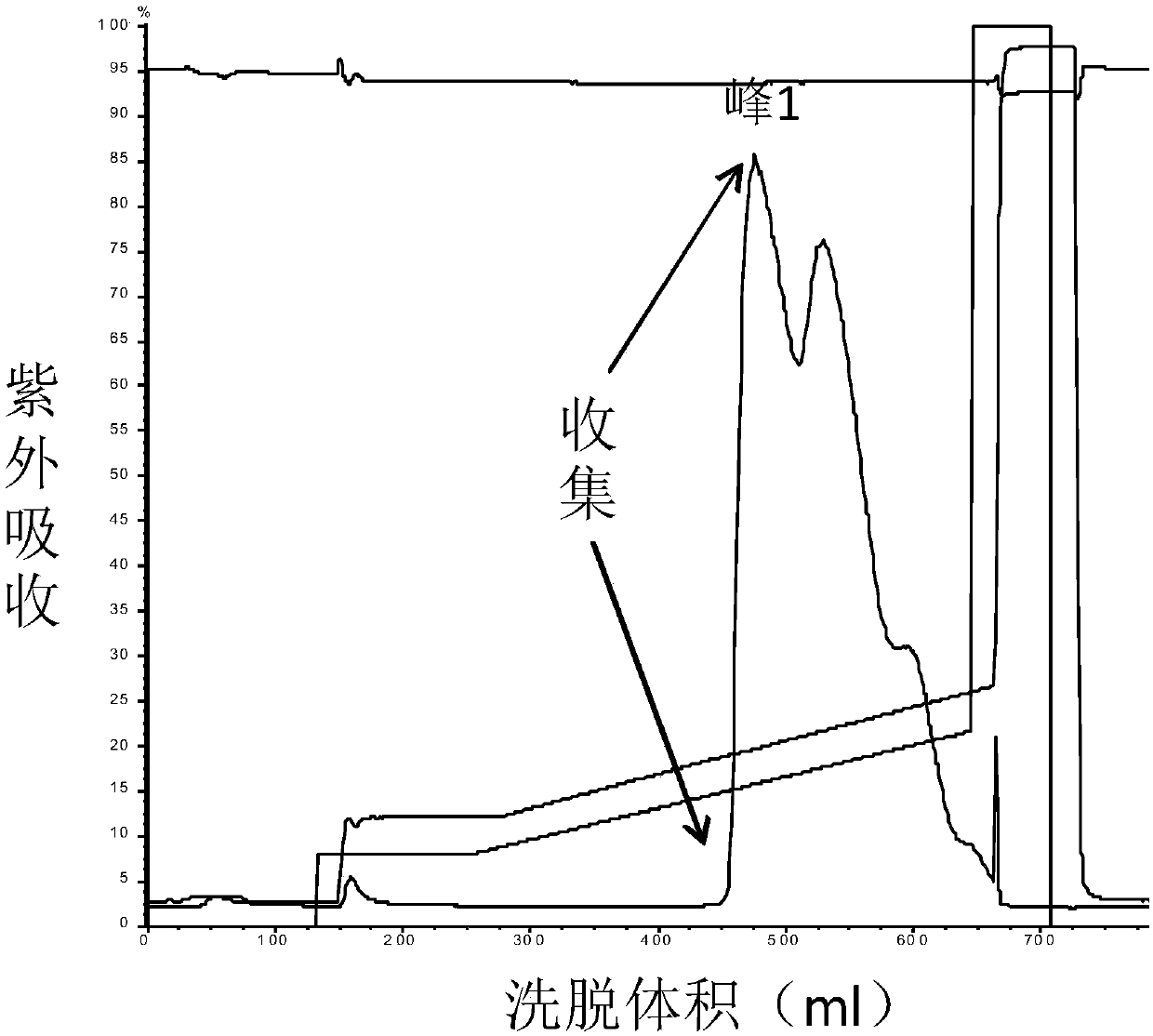
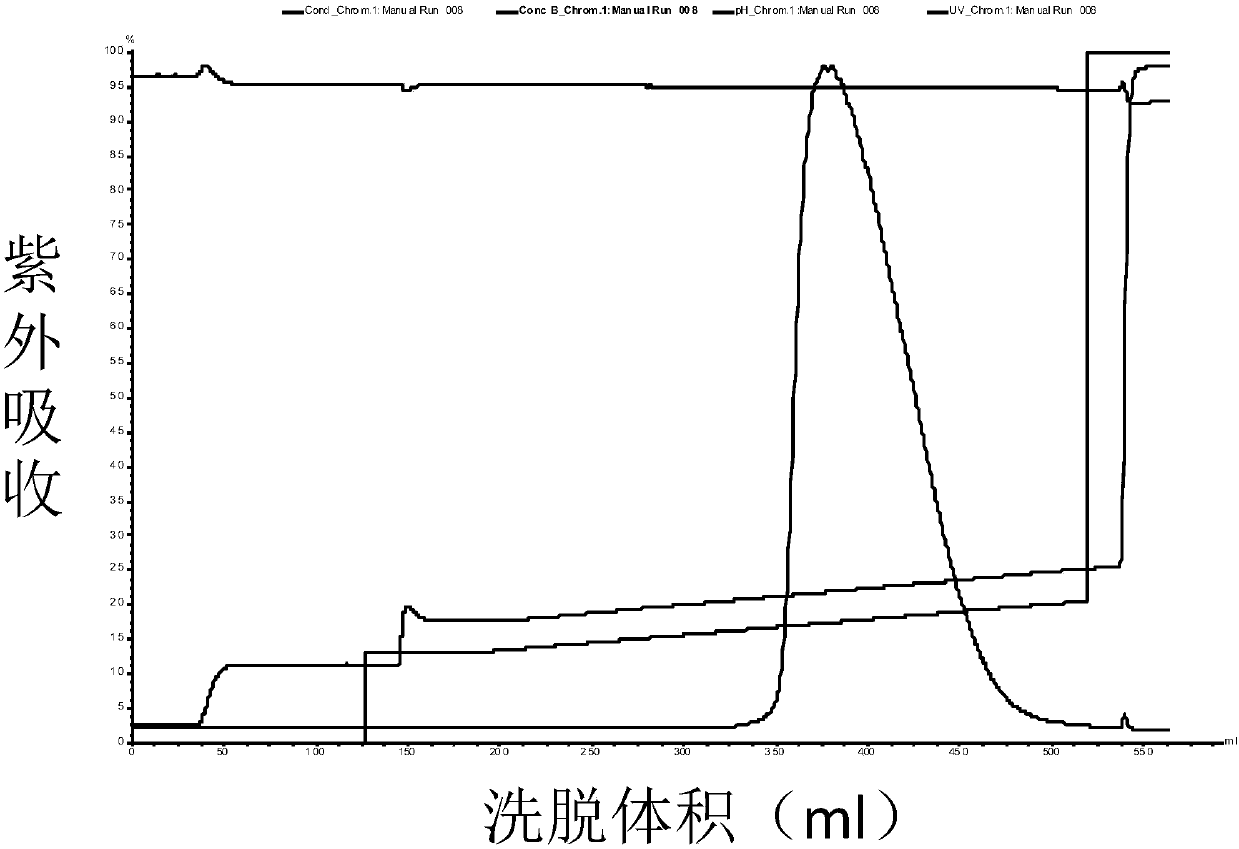
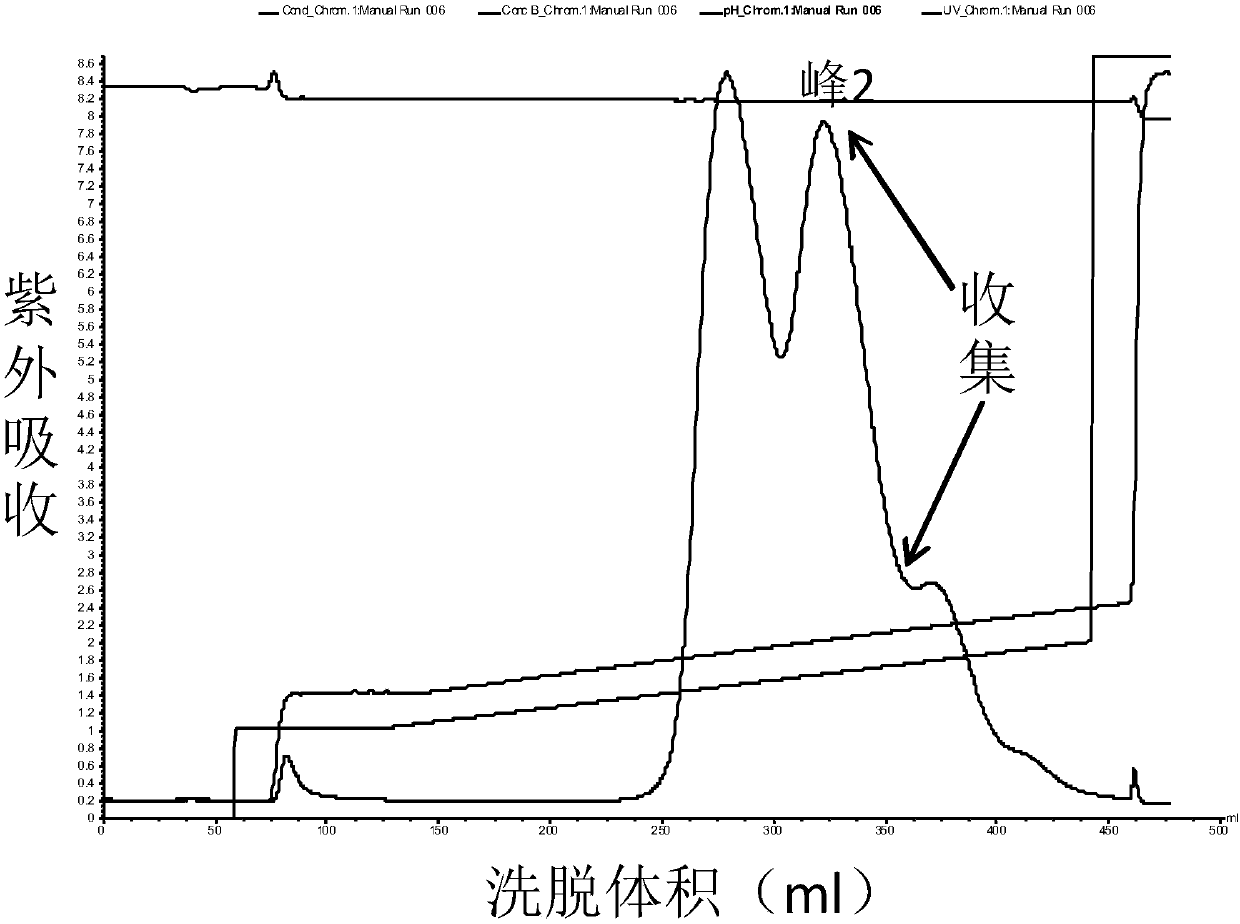
[0086] Collection: collect part of the eluted product before peak 1 as KLK1b, such as Figure 1a Shown; Partial elution product after peak 2 was collected as KLK1a, as Figure 2a As shown, the partially eluted product after peak 3 w...
Embodiment 2
[0098] Example 2 Detection of the purity of isolated products of porcine pancreatic kininogenase single components KLK1b, KLK1a and KLK1c
[0099] SDS-PAGE detection
[0100] (1) Electrophoresis: After preparing a 12% polyacrylamide gel, load and run. Run at 80V for 30min, and change to 150V for 30min after the bromophenol blue indicator runs out of the stacking gel.
[0101] (2) Coomassie Brilliant Blue staining: After electrophoresis, the gel was peeled off and placed in Coomassie Brilliant Blue staining solution for 30 minutes of staining.
[0102] (3) Decolorization: after dyeing, put the glue in the decolorization solution and decolorize overnight.
[0103] Analysis results such as Figure 4 shown. In terms of yield, the yield of KLK1b can reach more than 85%, the yield of KLK1a can reach 60%, and the yield of KLK1c is lower, but after separation and purification, the purity of each component of KLK1 can reach 95%. Therefore, when the purification method in this exam...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com