Ketoreductase dna molecule, recombinant vector and bacterial strain and application
A technology of DNA molecules and recombinant vectors, applied in the field of genetic engineering, can solve the problems of difficult industrialization, low specific enzyme activity, unstable expression, etc., achieve suitable scale production, large expression of enzyme activity, and avoid flammable and flammable The effect of explosive chemicals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment one provides a kind of step of constructing mutant genetically engineered bacteria of the present invention, as follows:
[0060] (1) Screening of mutant genes
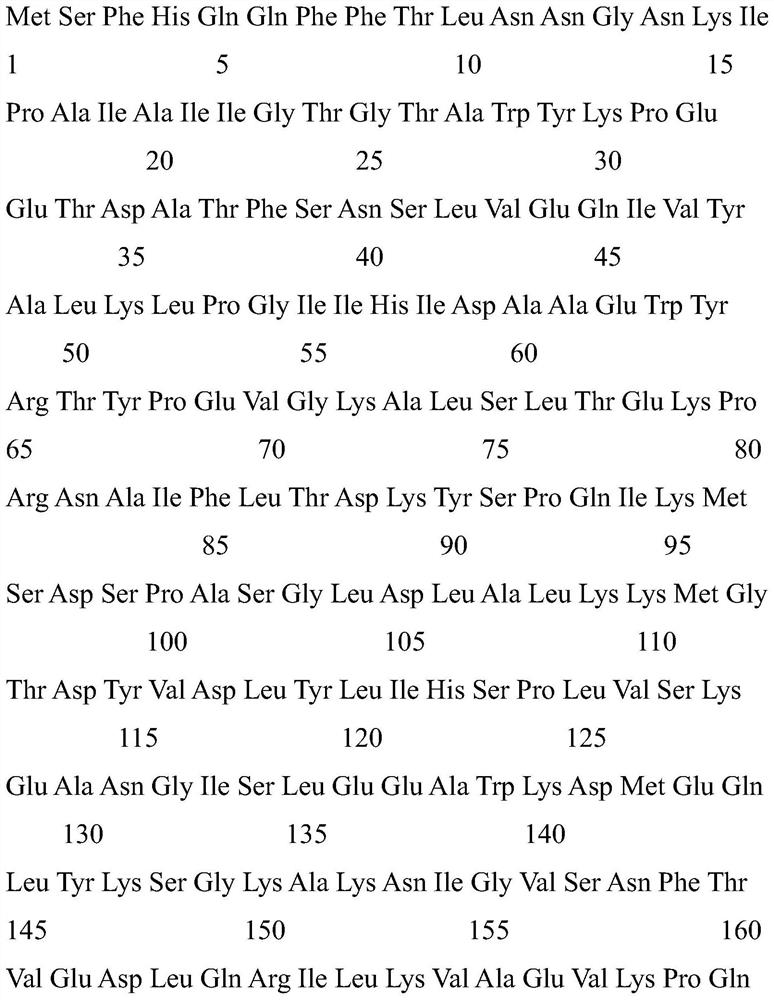
[0061] The 4H8N, 3WG6, and K amino acid sequences of ketoreductases from three different sources of wild fungus, Candida lipolytica, Candida portuguese, and Candida magnolia, were compared for homology comparison and analysis. The analysis of the hydrogen bond in the functional region, the binding site between the coenzyme and the ketoreductase, and the subunit interaction determined that the ketoreductase K sequence SEQ ID NO.3 of Candida magnolia was used as a template.
[0062] Wherein, the sequence of SEQ ID NO.3 is as follows:
[0063]
[0064]
[0065] Based on the ketoreductase K sequence SEQ ID NO.3, three amino acid mutation sites were selected through computer-aided semi-rational design, namely E102S (gag-tcc), L133I (tta-atc) and E172D (gaa-gat), that is, replace the 102nd glutamic...
Embodiment 2
[0125] Example 2 provides a comparison experiment of the specific enzyme activity of the ketoreductase produced by the strain prepared in Example 1 of the present invention and the ketoreductase produced by the original strain.
[0126] Among them, the specific enzyme activity refers to the unit of activity per mg of enzyme protein, which is the enzyme activity divided by the mass of enzyme protein.
[0127] Ligate the target fragment gene of known sequence and the gene fragment of the ketoreductase mutant with the double combination of amino acid sites in the 102 and 133 regions to the large fragment of the plasmid pBAD respectively, take 10 μL of the ligation reaction and add it to 100 μL of the sensory In the EP tube, mix gently in the EP tube. After 30 minutes of ice bath, heat shock at 42°C for 90 seconds. After taking it out, quickly ice bath for 2 minutes to cool the bacteria. Add 1 mL of antibiotic-free LB liquid medium (containing 10 μL of 10% arabinose inducer), shak...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com