Preparation method of water-soluble fluorescent calcium fluoride nanoparticle
A technology of fluorescent nanocrystals and calcium fluoride, applied in chemical instruments and methods, nanotechnology, nano-optics, etc., to achieve the effects of easy-to-obtain, low-cost, and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1) 0.01mol calcium nitrate is dissolved in 10 grams of deionized water to form solution A;
[0033] 2) 0.003mol potassium citrate and 0.001mol norleucine were dissolved in 10 grams of deionized water to form solution B;
[0034] 3) Dissolving 0.02mol sodium fluoride in 10 grams of deionized water to form solution C;
[0035] 4) Add the solution B obtained in step 2) to the solution A obtained in step 1) dropwise at room temperature, and fully stir for 15 minutes to form a mixed solution D;
[0036] 5) Add the solution C obtained in step 3) to the mixed solution D obtained in step 4) dropwise at room temperature, and fully stir for 15 minutes to form the mixed solution E;
[0037] 6) Transfer the mixed solution E obtained in step 5) to a closed hydrothermal reactor, react at 120° C. for 12 hours, and then naturally cool to room temperature to obtain a reaction solution;
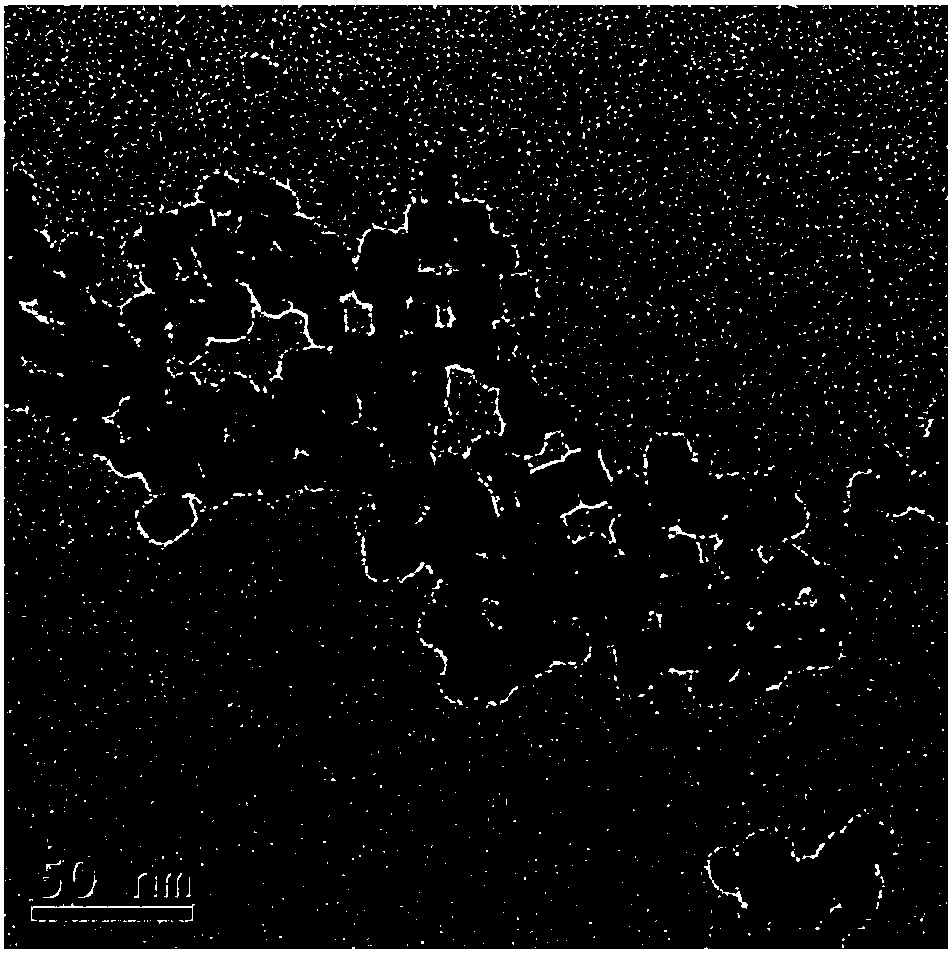
[0038] 7) Use a high-speed centrifuge to centrifuge the reaction solution obtained in step 6) to o...
Embodiment 2
[0039] Embodiment 2, with embodiment 1, difference is,
[0040] 1) 0.01mol calcium nitrate is dissolved in 10 grams of deionized water to form solution A;
[0041] 2) 0.0036mol sodium citrate and 0.001mol arginine were dissolved in 10 grams of deionized water to form solution B;
[0042] 3) Dissolving 0.0205 mol of potassium fluoride in 10 grams of deionized water to form solution C;
[0043] 6) Transfer the mixed solution E obtained in step 5) to a closed hydrothermal reactor, react at 150° C. for 9 hours, and then naturally cool to room temperature to obtain a reaction solution;
[0044] 7) Use a high-speed centrifuge to centrifuge the reaction solution obtained in step 6) to obtain a precipitate, alternately centrifuge and wash three times with deionized water and absolute ethanol to remove excess electrolyte in the system, then redisperse in deionized water, and adjust the pH to 8.5, to obtain water-soluble calcium fluoride fluorescent nanocrystals.
Embodiment 3
[0045] Embodiment 3, with embodiment 1, difference is,
[0046] 1) 0.01mol calcium chloride is dissolved in 10 grams of deionized water to form solution A;
[0047] 2) 0.015mol potassium citrate and 0.007mol norleucine were dissolved in 10 grams of deionized water to form solution B;
[0048] 3) Dissolving 0.0205 mol of potassium fluoride in 10 grams of deionized water to form solution C;
[0049] 6) Transfer the mixed solution E obtained in step 5) to a closed hydrothermal reactor, react at 180° C. for 3 hours, and then naturally cool to room temperature to obtain a reaction solution;
[0050] 7) Use a high-speed centrifuge to centrifuge the reaction solution obtained in step 6) to obtain a precipitate, alternately centrifuge and wash three times with deionized water and absolute ethanol to remove excess electrolyte in the system, then redisperse in deionized water, and adjust the pH to 9.5, to obtain water-soluble calcium fluoride fluorescent nanocrystals.
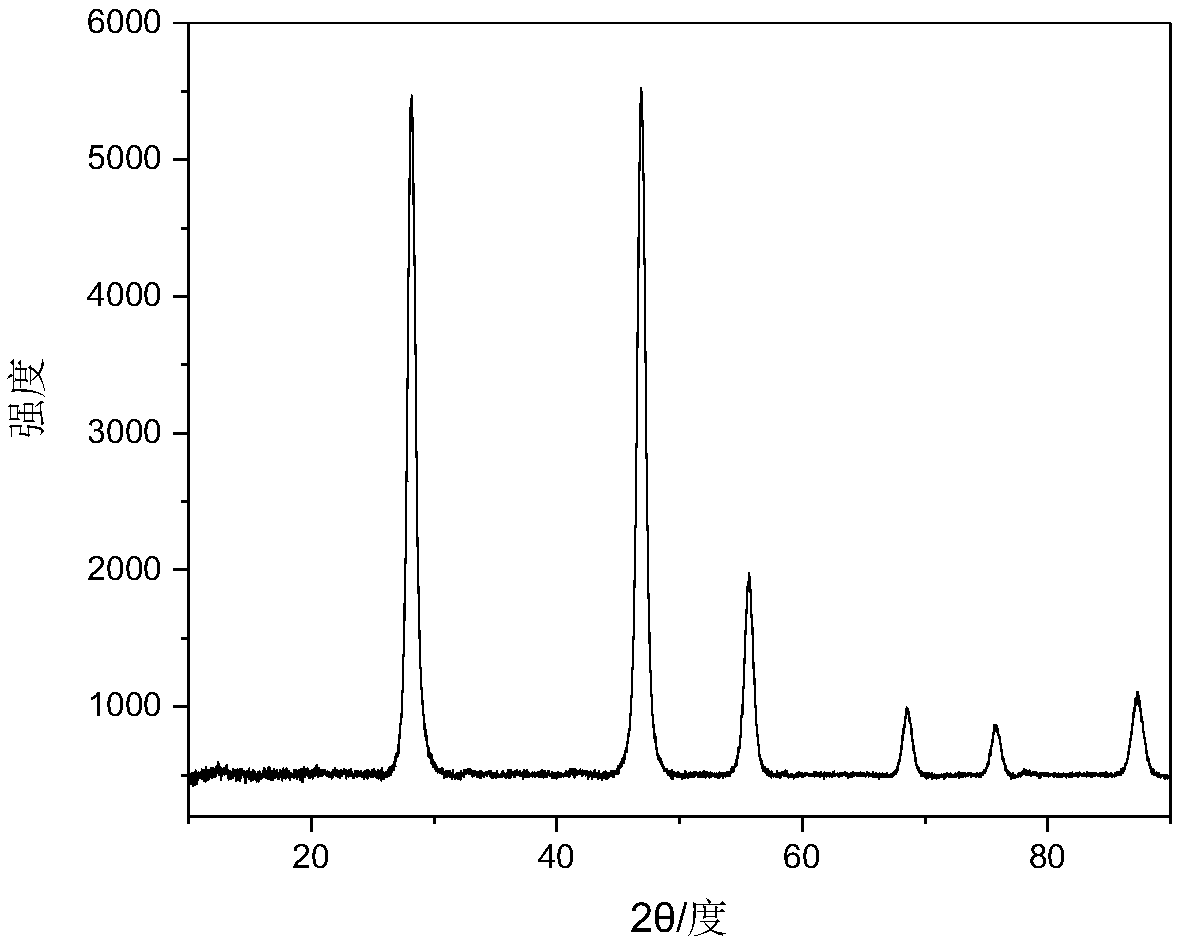
[0051] The X-...
PUM
| Property | Measurement | Unit |
|---|---|---|
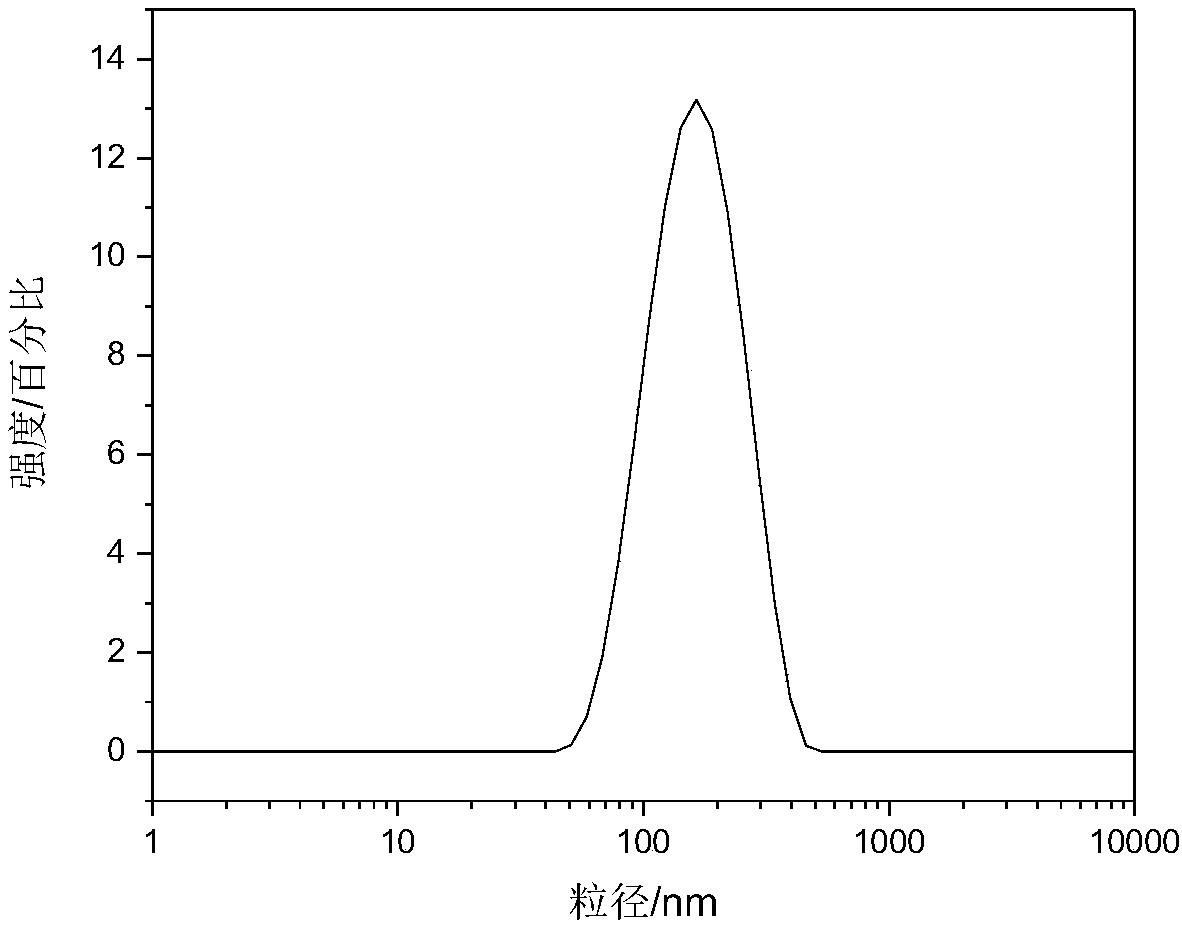
| Average size | aaaaa | aaaaa |
| Radius | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com