Preparation method of rivaroxaban tablets
A technology for tablet cores and blank tablet cores, which can be applied to medical preparations without active ingredients, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the problems of incomplete dissolution, slow disintegration, and plugging. Achieve the effects of rapid dissolution and absorption, simple preparation and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1. The prescription composition of rivaroxaban tablets: the specification is 20 mg, and the prescription quantity is 1000 tablets.
[0054]
[0055] The preparation steps of embodiment 1:
[0056] (1) Lactose, croscarmellose sodium, and microcrystalline cellulose of prescription quantity are mixed evenly;
[0057] (2) Magnesium stearate is mixed with the mixture of step 1, and compressed to obtain a blank tablet core;
[0058] (3) Prepare the rivaroxaban coating suspension as follows: using a suitable homogenizer, dissolve the prescribed amount of hypromellose, polyethylene glycol and sodium lauryl sulfate in an appropriate amount of purified water, and then dissolve the rivaroxaban The saban raw material is added to the above solution until the solid is evenly dispersed in the coating suspension;
[0059] (4) Fill an appropriate amount of blank tablet cores in step 2 into the coating machine to generate a fan-shaped spray to cover the entire width of th...
Embodiment 2
[0073] Example 2. The prescription composition of rivaroxaban tablets: the specification is 20 mg, and the prescription quantity is 1000 tablets.
[0074]
[0075]
[0076] Preparation method: with embodiment 1.
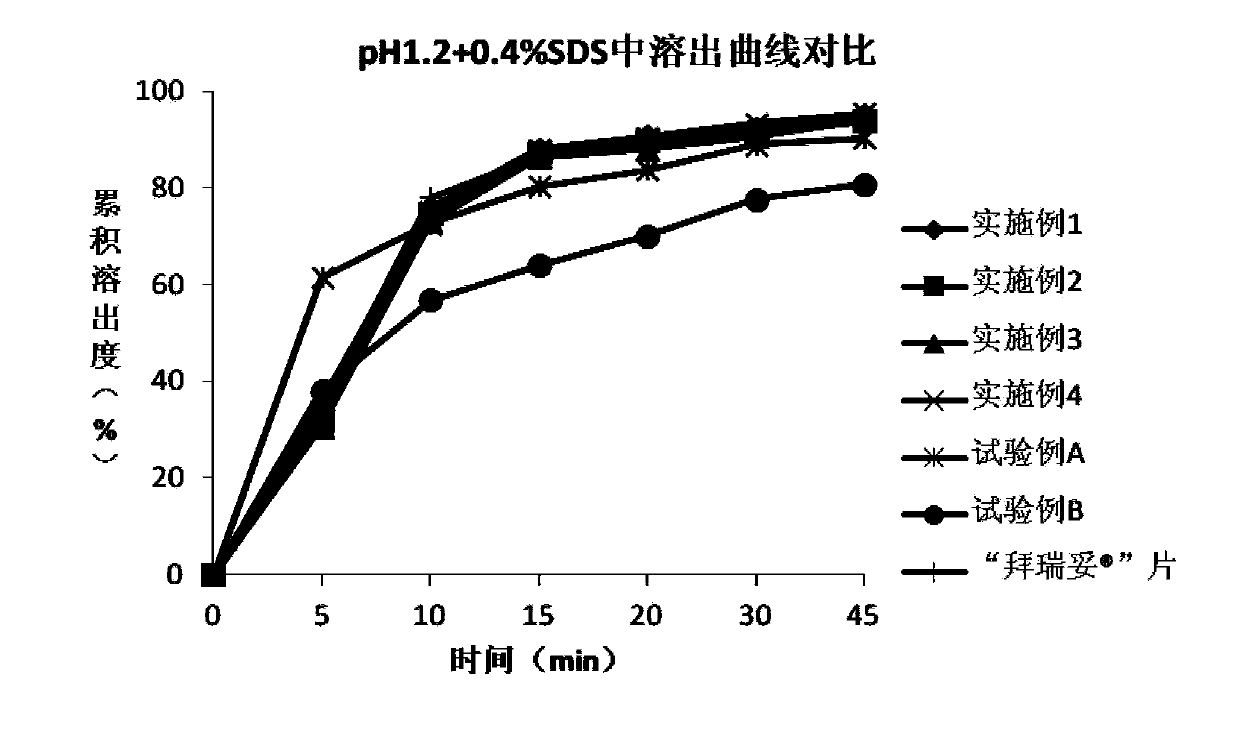
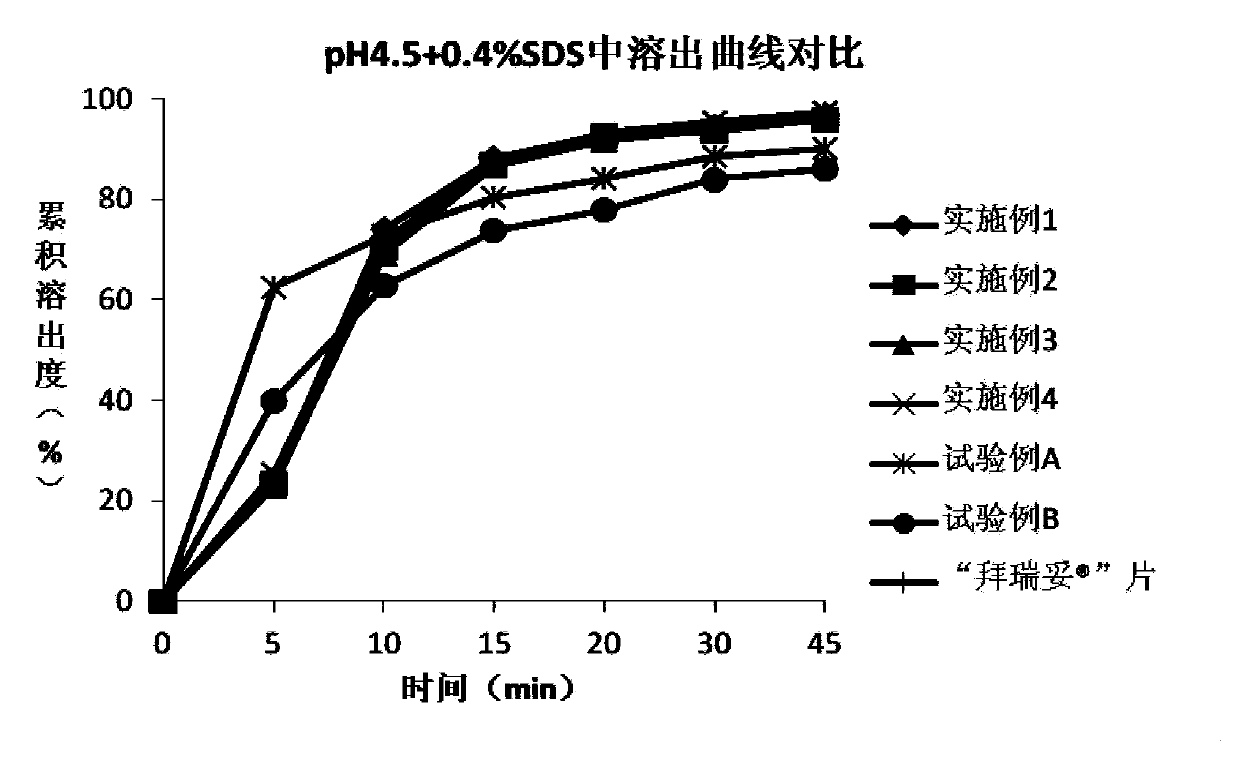
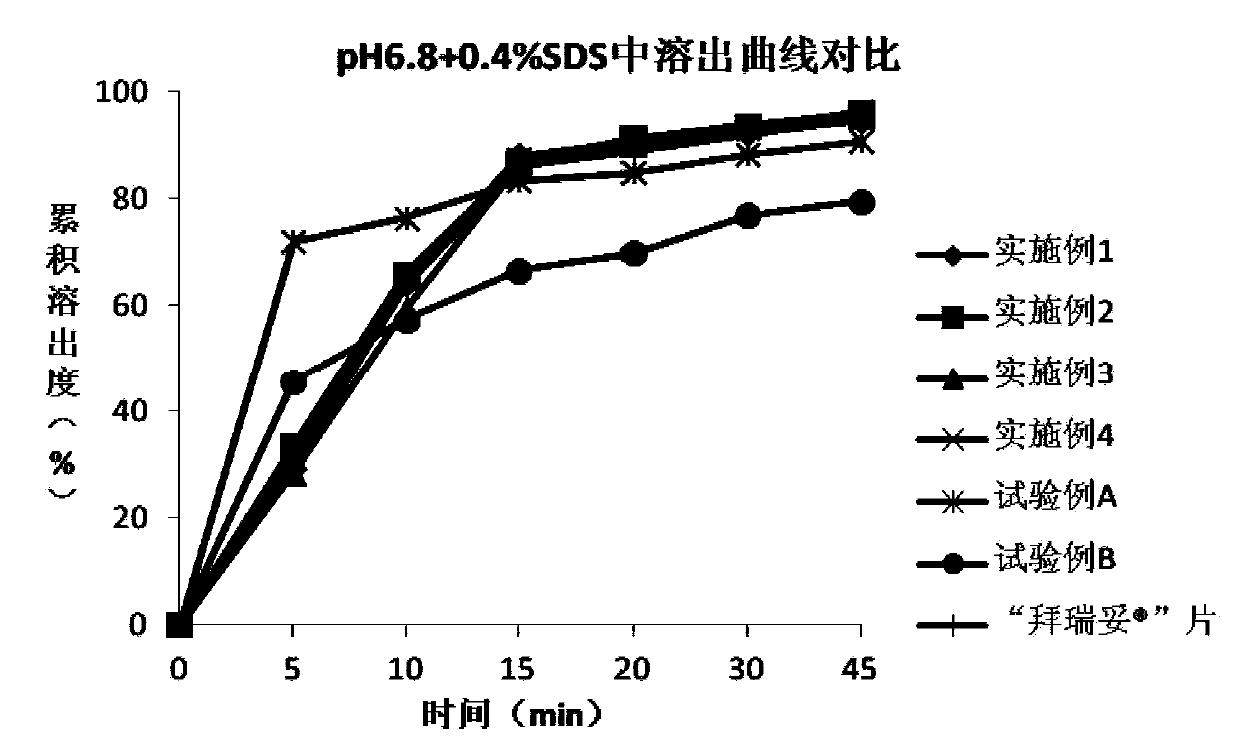
[0077] The rivaroxaban tablet of embodiment 2 and Comparison of dissolution curves of tablets in different dissolution media Figure 1~4 shown.
Embodiment 3
[0078] Example 3. The prescription composition of rivaroxaban tablets: the specification is 20 mg, and the prescription quantity is 1000 tablets.
[0079]
[0080] Preparation method: with embodiment 1.
[0081] The rivaroxaban tablet of embodiment 3 and Comparison of dissolution curves of tablets in different dissolution media Figure 1~4 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com