Preparation method and application of redox-sensitive induced pH-responsive nano drug carrier
A nano-drug carrier and nano-drug technology, which can be used in drug combinations, pharmaceutical formulations, medical preparations with inactive ingredients, etc., can solve the problems of fast systemic circulation clearance rate, poor targeting, and poor controlled release effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The synthesis of embodiment 1 monomer M1
[0026] The chemical structure of monomer M1 is shown in the figure below. The specific synthesis steps are as follows: First, add 2-aminoethyl methacrylate hydrochloride (1.65g, 10mmol) and triethylamine (2.02g, 20mmol) in a 100mL round bottom flask, add 50mL of anhydrous tetrahydrofuran to disperse Dissolve, add dropwise a solution of 2,4-dinitrobenzenesulfonyl chloride (2.66g, 10mmol) in tetrahydrofuran under ice-bath conditions, return to room temperature and react for 10h after the addition is complete. The reaction solution was distilled off the solvent under reduced pressure, and then separated by column chromatography, using ethyl acetate / petroleum ether mixture (common ratio) as the eluent, collecting the eluate containing the target compound, and after distilling off the solvent, to obtain The yield of the intermediate product 2-((2,4-dinitrophenyl)sulfonamide)ethyl methacrylate was 70%. Next, add 2-((2,4-dinitrophen...
Embodiment 2
[0030] The synthesis of embodiment 2 polymer P4
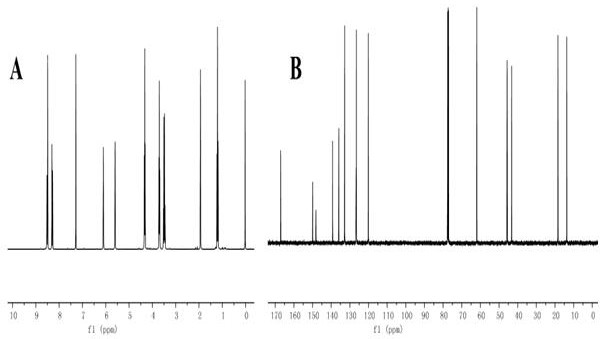
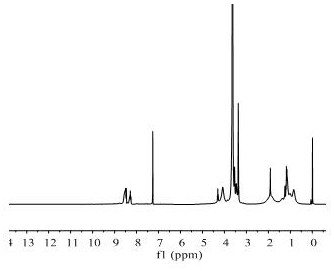
[0031]Add the reversible addition-fragmentation chain transfer polymerization (RAFT) chain transfer agent phenyl-(2-hydroxyethyl) thiocarbonate (6.1 mg, 0.1 mM) and polyethylene glycol monomethyl ether in sequence in the shlenk reaction tube Methyl methacrylate (mPEGMA) (500 mg, 1 mM) and 2-((2,4 dinitro-N-(ethyl) phenylsulfonamide) ethyl methacrylate (AMA-DNBSE) (455 mg, 1 mM ), initiator (AIBN) (1.64mg, 0.01mM) and 3mL solvent dimethylformamide (DMF), after three cycles of vacuum / argon, seal the reaction tube, and put it in an oil bath at 68-72℃ React for 24h. After the reaction, add DMF to dissolve, put it in a dialysis bag, dialyze with deionized water for 72h, and change the dialysate every 12h. After that, freeze-dry to obtain PEDF polymer. Utilize nuclear magnetic hydrogen spectrum to characterize P4 polymer , the result is attached figure 2 shown.
[0032] According to the method in embodiment 2, can obtain the poly...
Embodiment 3
[0035] The preparation method of embodiment 3 drug-loaded nanoparticles
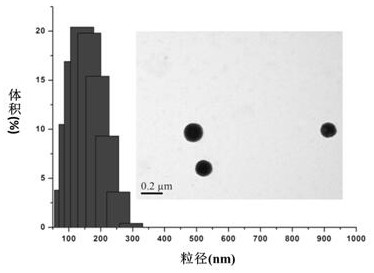
[0036] Weigh 20 mg of PEDF polymer and 6 mg of doxorubicin, ultrasonically dissolve them with 2 mL of trifluoroethanol, and disperse them directly in 10 mL of 0.01 M phosphate buffer solution with pH=7.4 under the action of ultrasonic to obtain N4 nanoparticles with a concentration of 2mg / mL. After the solvent evaporates completely, centrifuge to remove the deposited drug, and use the laser particle size analyzer and transmission electron microscope to detect the particle size and shape of the nanoparticles. The test results are shown in the attached image 3 As shown, the nanoparticles prepared in this example have a particle size of 120 nm, a particle size distribution of 0.12, and an obvious core-shell structure. The drug-loading amount of the nano-medicine detected by an ultraviolet spectrophotometer was 2.8%.
[0037] According to the preparation method in Example 3, drug-loaded nanoparticles with...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com