Matrine chitosan membrane and preparation method thereof
A matrine chitosan film and a technique for a matrine chitosan film are applied in the field of matrine chitosan film and its preparation, and can solve the problem that no relevant reports on matrine chitosan film have been seen, short half-life, Affecting drug efficacy and other problems, achieving the effect of simple and easy preparation method, good physical properties, and avoiding the use of organic solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The preparation of embodiment 1 film
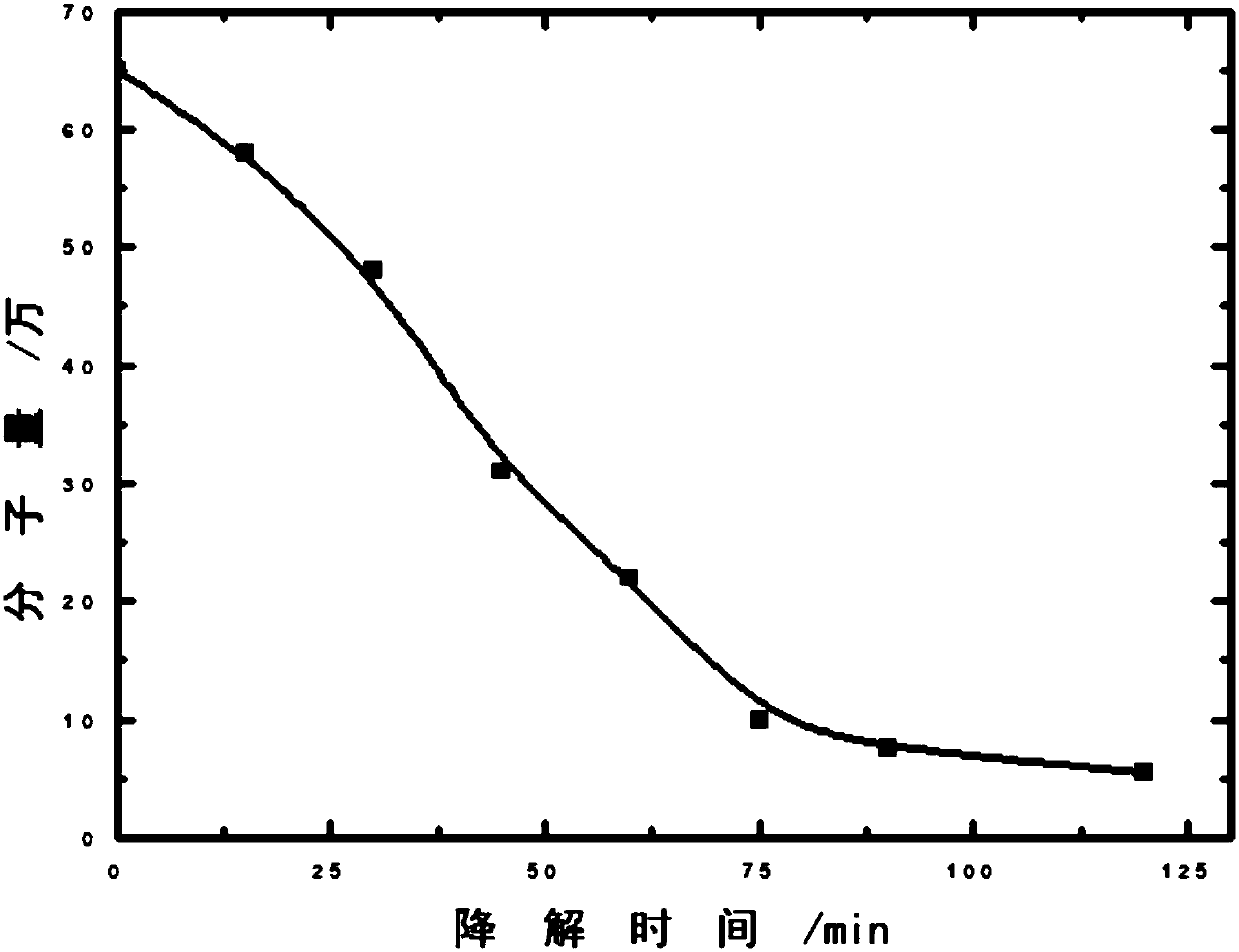
[0043] Configuration of CS solution: Dissolve CS in 1% (volume ratio) acetic acid solution to prepare 2L of 2% CS solution by mass. Pour the solution into a three-necked round-bottomed flask, and inject ozone O while stirring. 3 For degradation, take out about 250ml at the time of degradation of 0min, 15min, 30min, 45min, 60min, 75min, 90min, and 120min respectively.
[0044] Take 2.5ml of each of the above eight CS solutions with different degradation times, dissolve them in a mixed solvent of 0.1mol / l sodium acetate and 0.2mol / l acetic acid, and use a 50ml volumetric flask to make up a 1mg / ml CS solution.
[0045] With ozone (O 3 ) degradation method to degrade CS, the degradation of CS solution at room temperature at different times, and their viscosity-average molecular weight was measured by viscosity method. The relationship between CS viscosity-average molecular weight and degradation time is shown in Table 1, and the cha...
Embodiment 2
[0060] Select CSs with molecular weights of 310,000, 480,000, and 650,000, respectively, and make Mat / CS drug-loaded films with Mat contents of 10% and 12% (mass percent) according to the method in Example 1. After spraying gold, SEM observes its The surface morphology of the front and bottom surfaces; select CS with a molecular weight of 650,000, and make a Mat / CS drug-loaded film with a Mat content of 12% according to the method in Example 1, and observe its cross-sectional morphology by SEM after spraying gold.
[0061] The micro-morphological structure of the Mat / CS drug-loaded film was observed by scanning electron microscopy to understand the dispersion of plant-derived Mat drugs in the Mat / CS drug-loaded film. Figure 4-9 SEM pictures of Mat / CS drug-loaded membranes with different CS molecular weights and different Mat mass contents.
[0062] From Figure 4 It can be seen that the membrane front of the Mat / CS drug-loaded membrane ( Figure 4 B) No drug particles appea...
Embodiment 3
[0064] The in vitro release rate experiment of embodiment 3Mat / CS drug-loaded film
[0065] Accurately weigh the matrine reference substance, and put it in a measuring bottle to make a 0.2mg / ml solution. Draw 100, 200, 400, 600, 750, and 900 μl of solution with a microsampler, put them in a 60ml separating funnel, add 2×10 -4 mol / L bromothymol blue pH 7.6 buffer solution 10.00ml, chloroform 10.00ml, stopper and shake vigorously for 2min, let stand for about 2h, after the water layer and chloroform layer are completely separated, separate the chloroform layer. Absorbance was measured by UV spectrophotometer at a wavelength of 415 nm. When measuring, use 10.00ml of bromothymol blue buffer solution with a pH of 7.6, add 10.00ml of chloroform and shake as above, and separate the chloroform layer as a colorimetric blank. Use the measured absorbance as the ordinate and the concentration of matrine as the abscissa to measure the standard working curve of matrine. The fitted curve ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com