Method for preparing aromatic acid by direct carboxylation of CO2
A technology of aromatic acid and carboxylation method, which is applied in the field of CO2 direct carboxylation method to prepare aromatic acid, which can solve the problems of harsh operating conditions, high catalyst cost, and strong Lewis corrosion of equipment, so as to improve reaction selectivity and shorten reaction time , the effect of excellent solvency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] In the preparation method of the present invention: aromatic hydrocarbon is benzene, alkyl substituted benzene (alkyl substituted benzene is toluene, 1,2-xylene, 1,3-xylene, 1,4-xylene, 1,3,5- trimethylbenzene, ethylbenzene, propylbenzene or tert-butylbenzene), halogenated benzene, naphthalene or alkyl-substituted naphthalene;
[0040] Lewis acid is AlCl 3 , AlBr 3 , FeCl 3 , FeBr 3 , BF 3 , SbF 5 , NbCl 5 and La(CF 3 SO 3 ) 3 One of, preferably AlCl 3 , less preferably AlBr 3 , FeCl 3 or FeBr 3 ;
[0041] Organic bases include alkylamines (alkylamines are primary amines R-NH 2 , secondary amine R 1 R 2 -NH or tertiary amine R 1 R 2 R 3 -N, where R 1 , R 2 and R 3 Both are C 1 ~C 18 Alkyl, allyl and various secondary or tertiary alkyl groups), imidazole / N-alkyl substituted imidazole (in imidazole / N-alkyl substituted imidazole, the alkyl is C 1 ~C 18 Alkyl, allyl and various secondary or tertiary alkyl groups), pyridine / alkyl substituted pyridin...
Embodiment 1
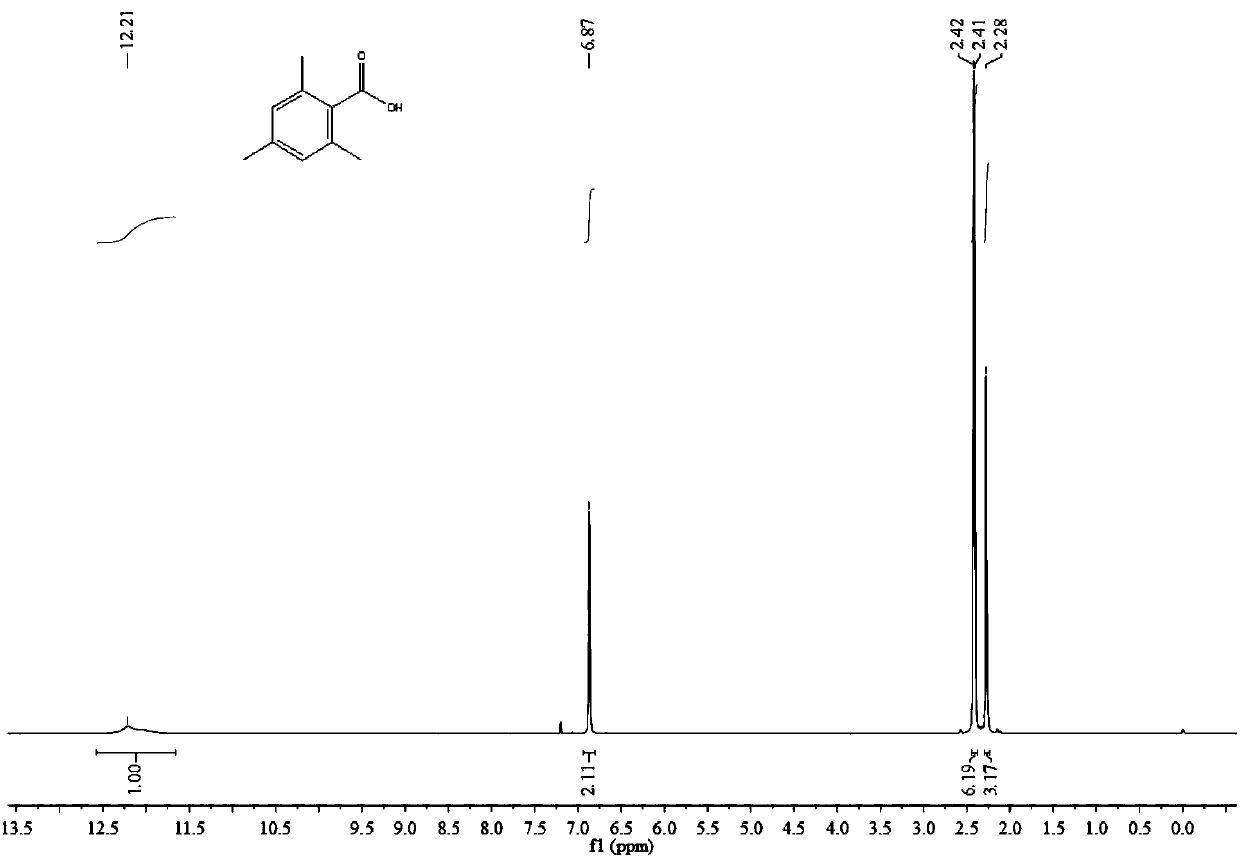
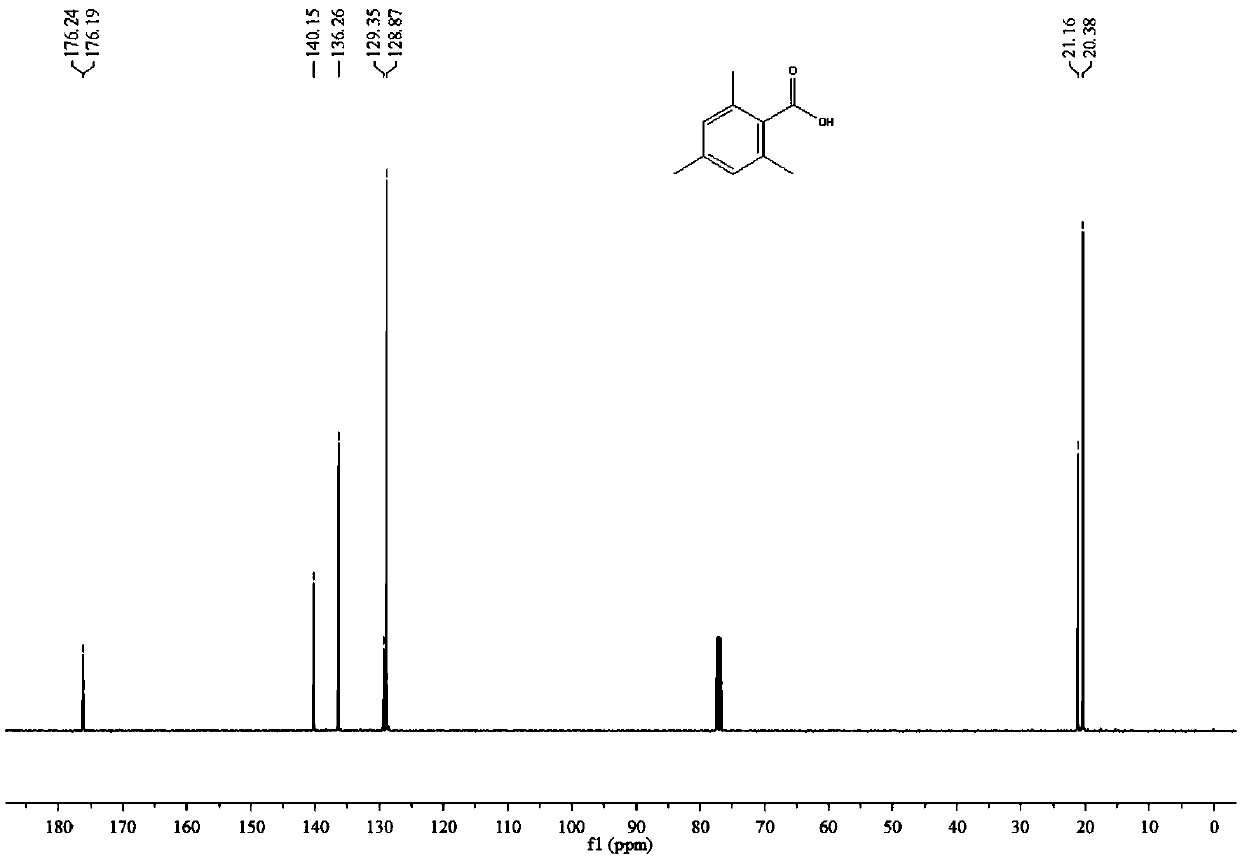
[0043] Under argon atmosphere, quickly add 40mL of dry 1,3,5-trimethylbenzene and 2.5g of anhydrous AlCl to a 250mL autoclave lined with PTFE 3 , 1.76g of dry N-methyldicyclohexylamine, and the reaction kettle was sealed after the feeding was completed. Then CO 2 The cylinder is connected to the autoclave through the pipeline, and the valve is opened to control the CO 2 The pressure was 6MPa, and at the same time, the stirring was started at a stirring rate of 1000rpm, and finally heating was started and kept at 40°C for 30h. After the reaction was completed, 150 mL of water was added to the reaction system, and the reaction was carried out for 30 min under stirring conditions, and then extracted three times with 50 mL of ether, and the extracts were combined and concentrated to dryness to obtain 2.72 g of off-white solid. Dissolve the above-mentioned off-white solid in 20mL of 10%wt sodium hydroxide solution, filter the insoluble matter to obtain the filtrate, then adjust t...
Embodiment 2
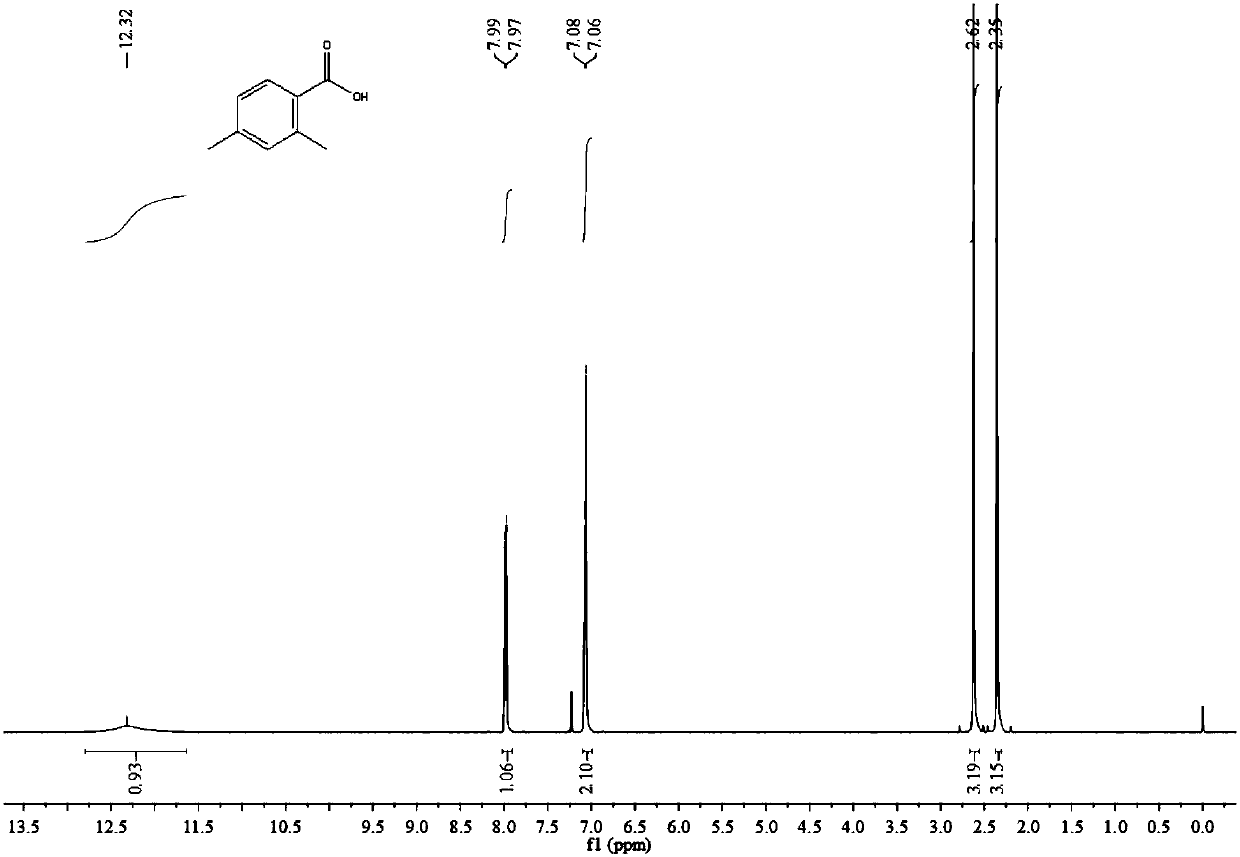
[0045] Under argon atmosphere, quickly add 40mL of dry 1,3,5-trimethylbenzene and 2.5g of anhydrous AlCl to a 250mL autoclave lined with PTFE 3 , 0.54g of dry 1-allylimidazole, and the reaction kettle was sealed after the feeding was completed. Then CO 2 The cylinder is connected to the autoclave through the pipeline, and the valve is opened to control the CO 2 The pressure was 6MPa, and at the same time, the stirring was started at a stirring rate of 1000rpm, and finally heating was started and kept at 40°C for 24h. After the reaction was completed, 150 mL of water was added to the reaction system, and the mixture was reacted for 30 min under stirring, and then extracted three times with 50 mL of ether. The extracts were combined, concentrated and dried to obtain 2.85 g of off-white solid. Dissolve the above-mentioned off-white solid in 20mL of 10%wt sodium hydroxide solution, filter the insoluble matter to obtain the filtrate, then adjust the pH value of the filtrate to 1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com