Method for separating and measuring related impurities in acrivastine and preparation thereof
A technology of impurities and preparations, applied in the field of analytical chemistry, to achieve high sensitivity, excellent separation performance and durability, and short separation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Method for Separating and Determining Avastatin and Its Related Impurities
[0046] 1) Protect from light, take an appropriate amount of Avastin, add mobile phase to dissolve and dilute to make a solution containing about 200 μg of Avastin per 1ml, as the test sample solution; accurately measure 1ml, put it in a 100ml measuring bottle , dilute to the mark with mobile phase, shake well, and use it as a control solution.
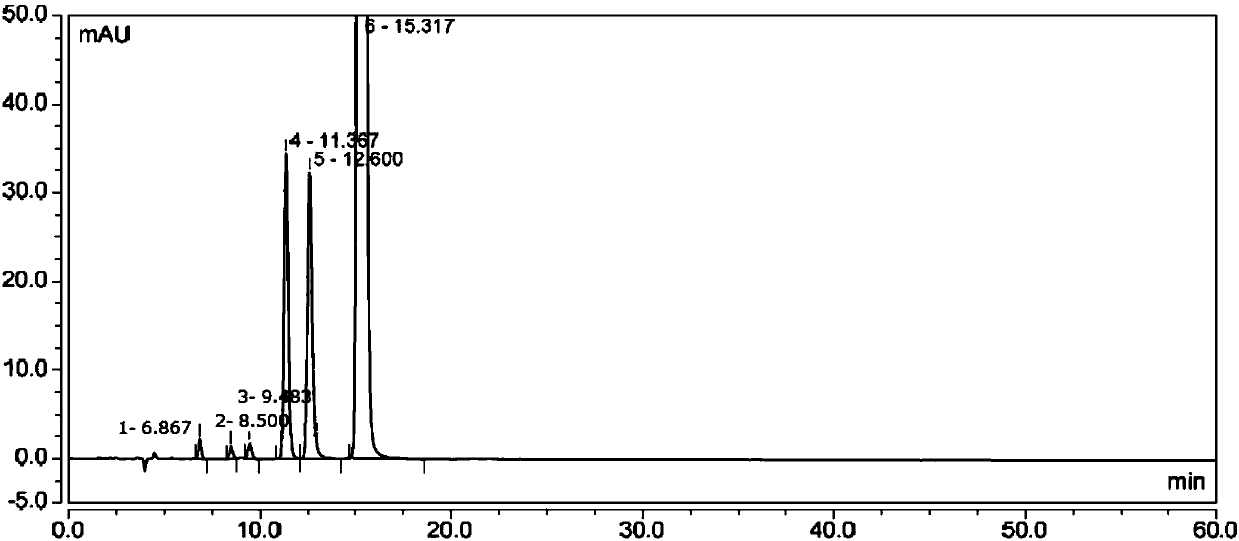
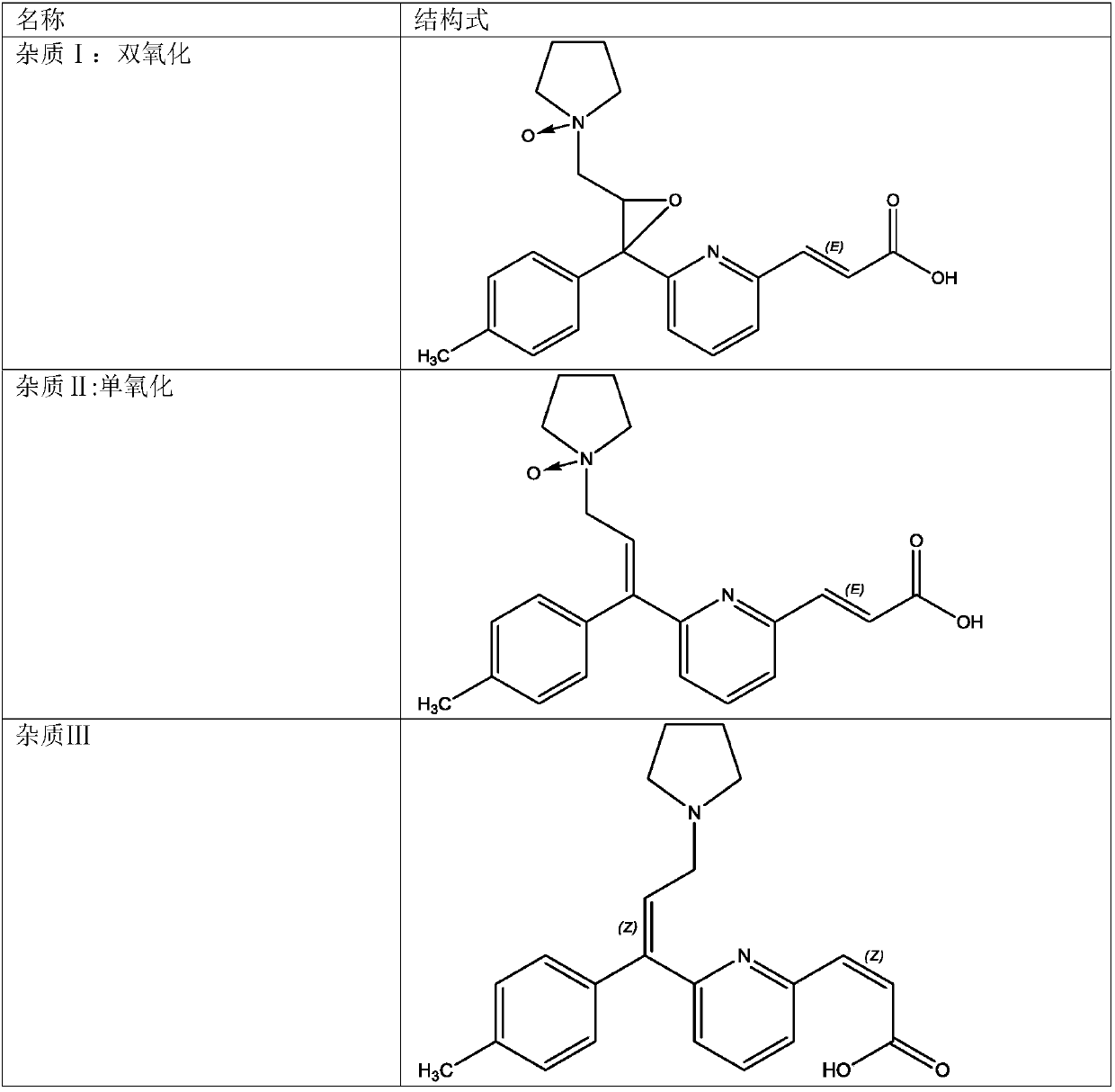
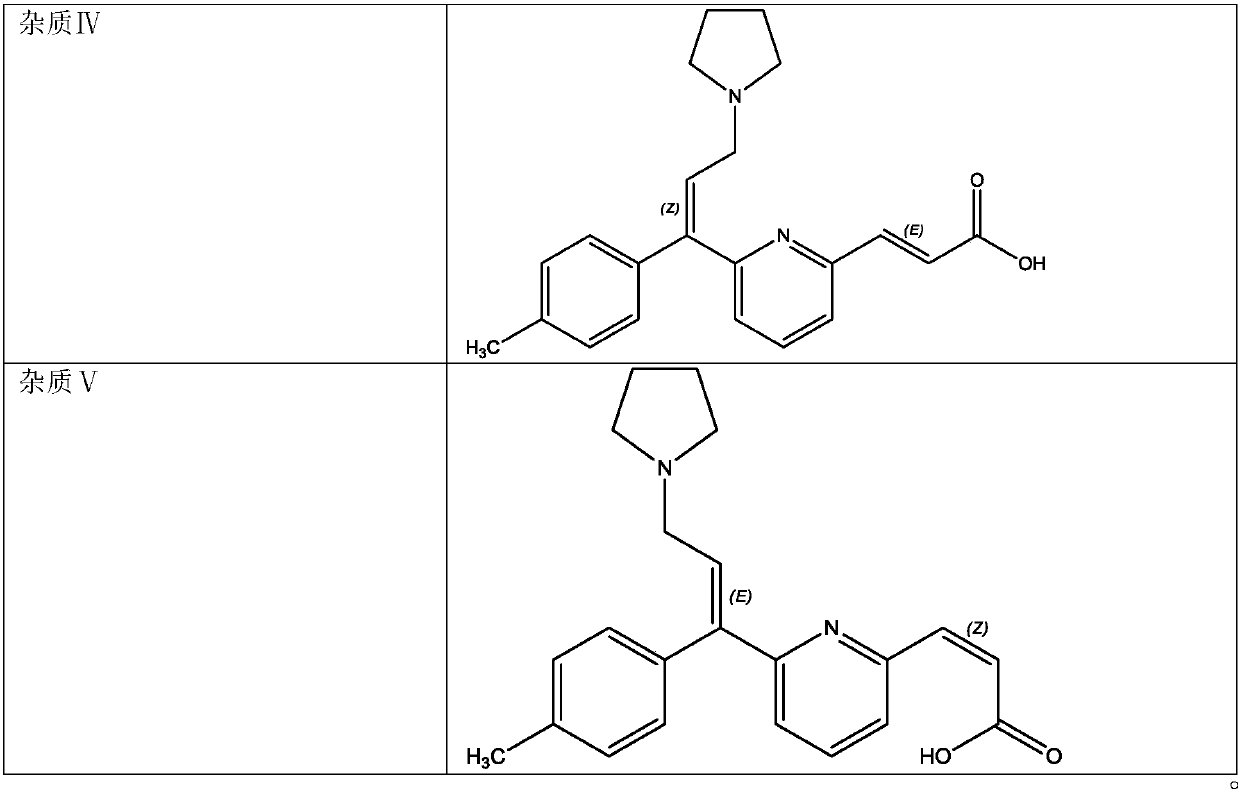
[0047] 2) Take an appropriate amount of Avastin reference substance, put it in a quartz volumetric flask, add mobile phase to ultrasonically dissolve and quantitatively dilute to make a solution containing about 200 μg of Avastin per 1 ml, and light it in a 5000 lx light box for 48 hours as a system adaptability Solution, take 20 μl into the liquid chromatograph, record the chromatogram, the order of peaks is impurity Ⅰ, impurity Ⅱ, impurity Ⅲ, impurity Ⅳ, impurity Ⅴ and Avastin peak, the number of theoretical plates is calculated according to...
Embodiment 2
[0050] Embodiment 2 Separation and determination of Avastin capsules and the method for its related impurities
[0051] 1) Protect from light, take an appropriate amount of the content of Avastin capsules (approximately equivalent to 20mg of Avastin), put it in a 100ml brown measuring bottle, add mobile phase ultrasound and shake for 10 minutes from time to time to make Avastin Tin dissolved, and diluted to the mark with the mobile phase, shaken, filtered, and the filtrate was used as the test sample solution; 1ml was accurately measured, put in a 100ml measuring bottle, diluted to the mark with the mobile phase, shaken up, as a contrast solution.
[0052] 2) Take an appropriate amount of Avastin reference substance, put it in a quartz volumetric flask, add mobile phase to ultrasonically dissolve and quantitatively dilute to make a solution containing about 200 μg of Avastin per 1 ml, and light it in a 5000 lx light box for 48 hours as a system adaptability Solution, take 20 μl ...
Embodiment 3
[0055] Example 3 Method for Separating and Determining Avastin Capsules and Related Impurities
[0056] Except that the mobile phase proportioning that adopts is different, all the other conditions are identical with embodiment 2.
[0057] The volume ratio of acetonitrile, tetrahydrofuran and 0.5% triethylamine aqueous solution in the mobile phase is 15:5:80.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com