Blocking method for activated quantum dots
A technology of quantum dots and mass ratio, which is applied in the closed field of activated quantum dots, can solve the problems of reducing reagent stability and sensitivity, inert protein shedding, and difficulty in completely covering the exposed areas of activated quantum dots, so as to improve sensitivity and stability, Effects of improving stability and increasing sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Preparation of Quantum Dot Antibody Complex and Test of Coupling Amount
[0028] 1. Activation and coupling of quantum dots (QDs)
[0029] Take 50 μl of QDs stock solution with a solid content of 10%, dilute to 500 μl with 50 mM boric acid-borax buffer (pH 9.0), mix well, add 50 μl EDC solution (5 mg / ml), 5 μl NHS solution (75 mg / ml), mix well Stir and react at room temperature (25°C) for 30 minutes, centrifuge and wash once after incubation, and resuspend with 500 μl of 20 mM HEPES buffer (pH 7.0), take 75 μg of L-1# antibody, add it to the QDs resuspension, mix After uniformity, stir at room temperature for 1 hour;
[0030] 2. Processing of BSA
[0031] A 20% BSA solution and a 2% EDC solution were prepared with blocking buffer (20mM HEPES buffer, pH 7.0), and the two solutions were mixed 1:1 and stirred at 4°C for 2 hours.
[0032] 3. Closed
[0033] Add 500 μl of poly-BSA solution to the system after the reaction in step 1 to resuspend, mix well, stir ...
Embodiment 2
[0034] Example 2 Preparation and Specific Detection of Lipoprotein Phospholipase A2 (Lp-PLA2) Quantum Dot Immunofluorescence Detection Kit
[0035] 1. Preparation of QDs-L-1# labeling complex
[0036]Prepare the QDs-L-1# labeling complex according to steps 1-4 in Example 1;
[0037] 2. Treatment of binding pads and immobilization of labeling complexes
[0038] Cut the binding pad into a width of 8mm and soak it in citric acid-sodium citrate buffer (pH8.0, 20mM) containing 5% trehalose and 1% Tween 20 for 5 minutes, and dry it at room temperature. The bonding pad is placed in a constant temperature blast drying oven, and dried at 37°C for 16 hours;
[0039] Take out the dried conjugation pad and fix the labeling complex prepared in step 1 on the conjugation pad with a gold sprayer at a spray volume of 5 μl / cm, place it in a constant temperature blast drying oven, and dry it at 37°C for 16 hours.
[0040] 3. Fixation of T-line and C-line on nitrocellulose membrane
[0041] D...
Embodiment 3
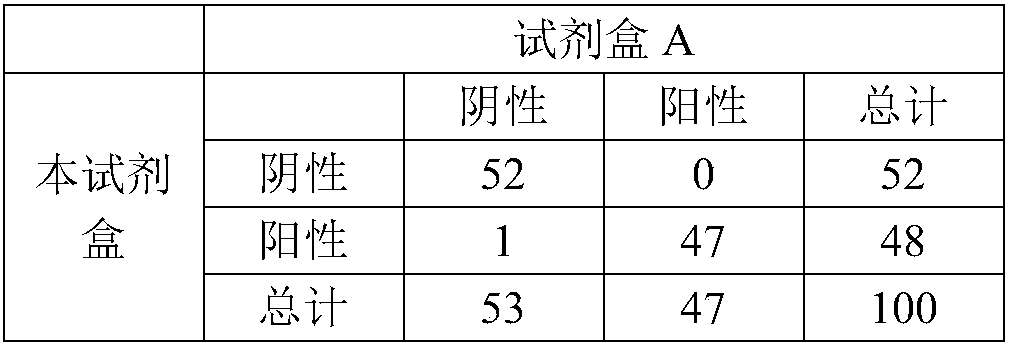
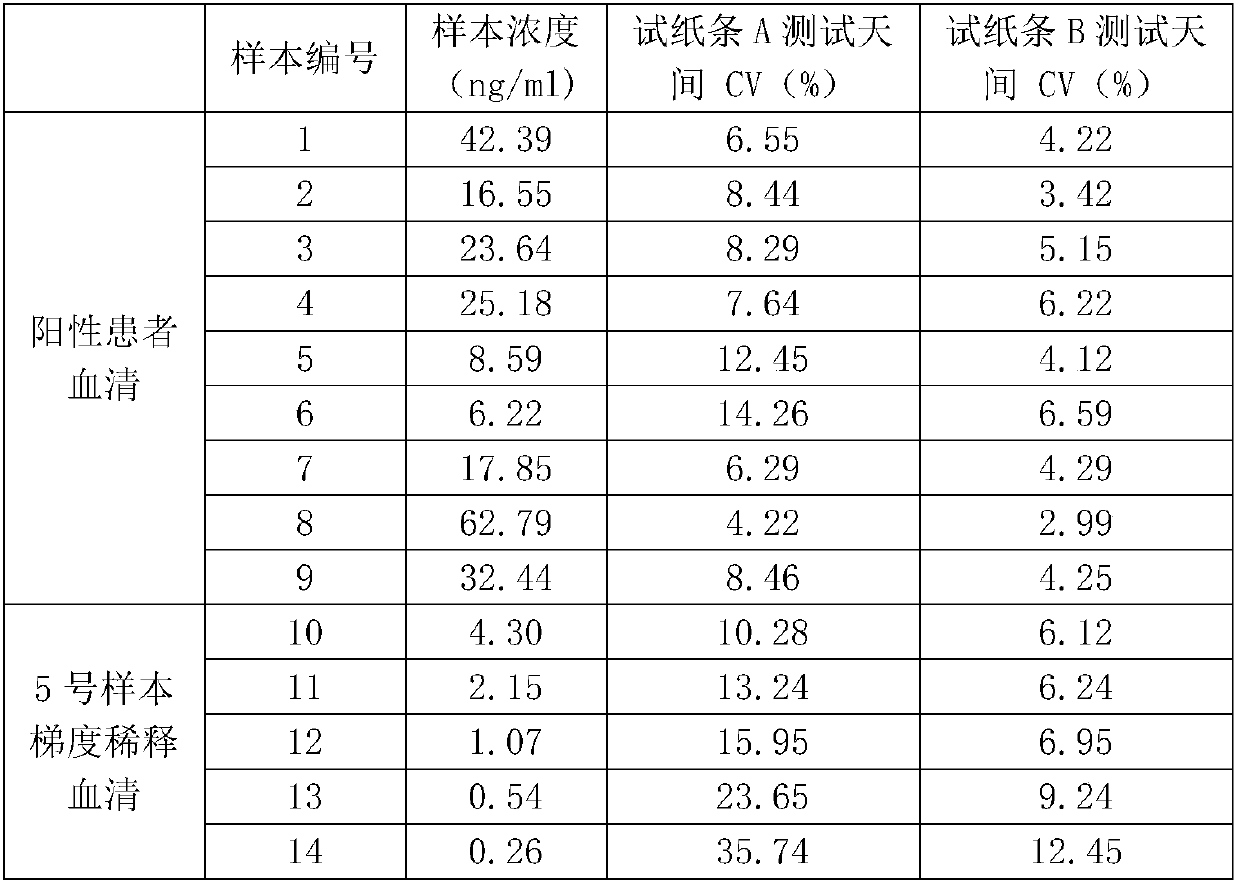
[0054] Example 3 Preparation, Sensitivity and Stability Detection of Heart-type Fatty Acid Binding Protein (H-FABP) Quantum Dot Immunofluorescence Detection Kit
[0055] 1. Preparation of QDs-FP1# labeling complex by prior art
[0056] Take 50 μl of QDs stock solution with a solid content of 10%, dilute to 500 μl with 50 mM boric acid-borax buffer (pH 9.0), mix well, add 5 μl EDC solution (50 mg / ml), 5 μl NHS solution (75 mg / ml), mix well Stir the reaction at room temperature (25°C) for 30 minutes, centrifuge and wash once after incubation, and resuspend with 500 μl of 20mM MOPS buffer (pH 6.5);
[0057] Take 75 μg of FP1# antibody, add it to the QDs resuspension in step (1), mix well, stir at room temperature for 1 hour, centrifuge and wash once after the reaction is completed, and add 500 μl of blocking solution (20% BSA) to resuspend After mixing, stir and react at room temperature for 1 hour. After the reaction is completed, centrifuge and wash once, resuspend with 500 μl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com