CEA (carcinoembryonic antigen) resistant nanomater antibody with high appetency and application
A nano-antibody and affinity technology, which is applied in the fields of application, antibody, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc. It can solve the problems of short serum half-life, low affinity, easy aggregation, etc., and achieve superior application Effects of foreground, high specificity, and sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1. Construction and screening of anti-CEA nanobody phage display library
[0031] 1.1 Immunization of alpaca: Select a healthy adult alpaca, mix the recombinant protein CEA and Freund's adjuvant at a ratio of 1:1, and immunize the alpaca by subcutaneous multi-point injection on the back at 6-7μg / Kg , a total of four immunizations, immunization interval of 2 weeks. Afterwards, the peripheral blood of the alpaca was collected for the construction of a phage display library.
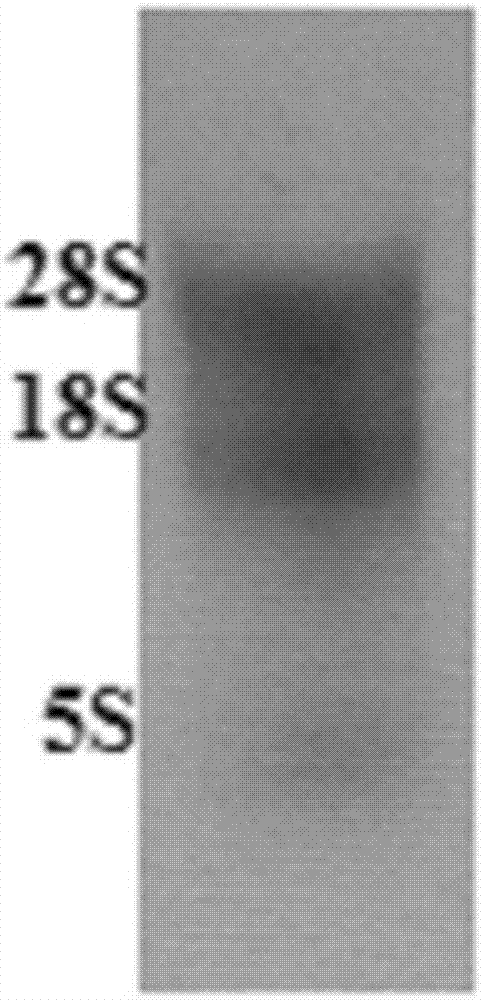
[0032] 1.2 Separation of camel-derived lymphocytes: analyze lymphocytes from the collected camel-derived anticoagulated whole blood according to routine procedures in this technical field, every 2.5×10 7 Add 1mL RNA isolation reagent to each living cell, take 1mL for RNA extraction, and store at -80°C.
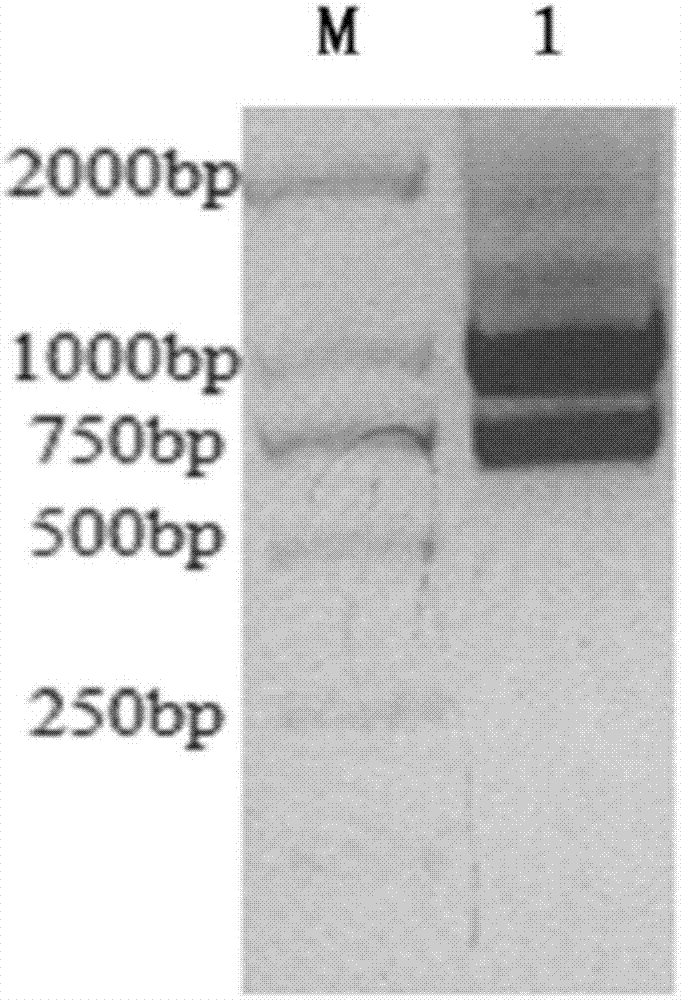
[0033] 1.3 Extraction of total RNA: extract total RNA according to routine procedures in this technical field, and adjust the concentration to 1 μg / μL with RNase-free water (for the identif...
Embodiment 2
[0061] Example 2. Human kidney epithelial cell line transfected with adenovirus E1A gene expresses fusion nanobody 2D5-HAP
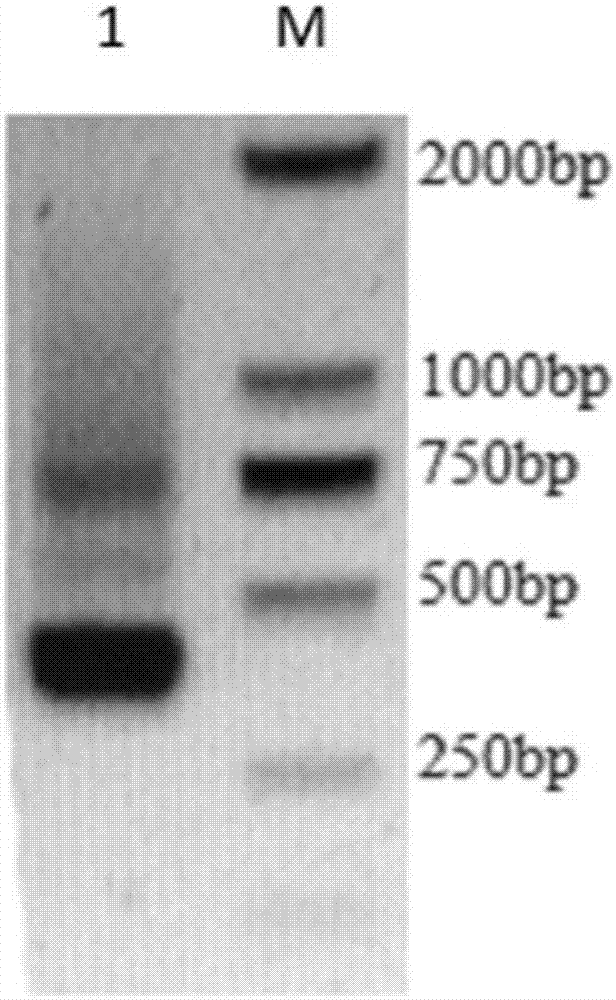
[0062] The binding site sequence of human alkaline phosphatase is used as the amino acid sequence of the chemiluminescent region (shown in SEQ ID NO.5), which is fused with the flexible polypeptide and the 2D5 nanobody to form a nanobody with a chemiluminescent region sequence, and synthesized The nucleotide coding sequence of the fusion protein (shown in SEQ ID NO.6), two restriction sites HindⅢ and EcoRI are added at the two ends of the coding sequence of the nucleotide, and the two restriction sites The point ligated to the vector pcDNA3.1(+). 293 cells in logarithmic growth were used for transfection after the plasmid was extracted without endotoxin. After the transfected cells were cultured for 36 hours, the cell culture medium was poured into a 50ml centrifuge tube, centrifuged at 12000g for 5 minutes, the supernatant was collected, filtered with ...
Embodiment 3
[0063] Example 3.2D5-HAP affinity activity with CEA antigen
[0064] 3.1 Chip antigen coupling
[0065] The antigen was prepared into a 20 μg / mL working solution with different pH sodium acetate buffers (pH 5.5, pH 5.0, pH 4.5, pH 4.0), and a 50 mM NaOH regeneration solution was prepared at the same time, and the Biacore T100 protein interaction analysis system was used in the instrument The template method is used to analyze the electrostatic binding between the antigen and the surface of the chip (GE company) under different pH conditions, and the signal increase amount reaches 5 times RL as the standard, select the most suitable neutral pH system and adjust it as needed The antigen concentration was used as the condition during coupling. The chip was coupled according to the template method that comes with the instrument: select the blank coupling mode for channel 1, select the Target coupling mode for channel 2, and set the target to the designed theoretical coupling amou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com