Rhombic cell containing spiral rings and synthesis method thereof
A synthesis method and cell technology, applied in the fields of nanotechnology and organic electronics, can solve the problems of toxic post-processing, large pollution, complex synthesis steps, etc., and achieve the effects of simple post-processing, high yield, and mature reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0045] Example 1: Preparation of Rhombus Cell-2a
[0046]
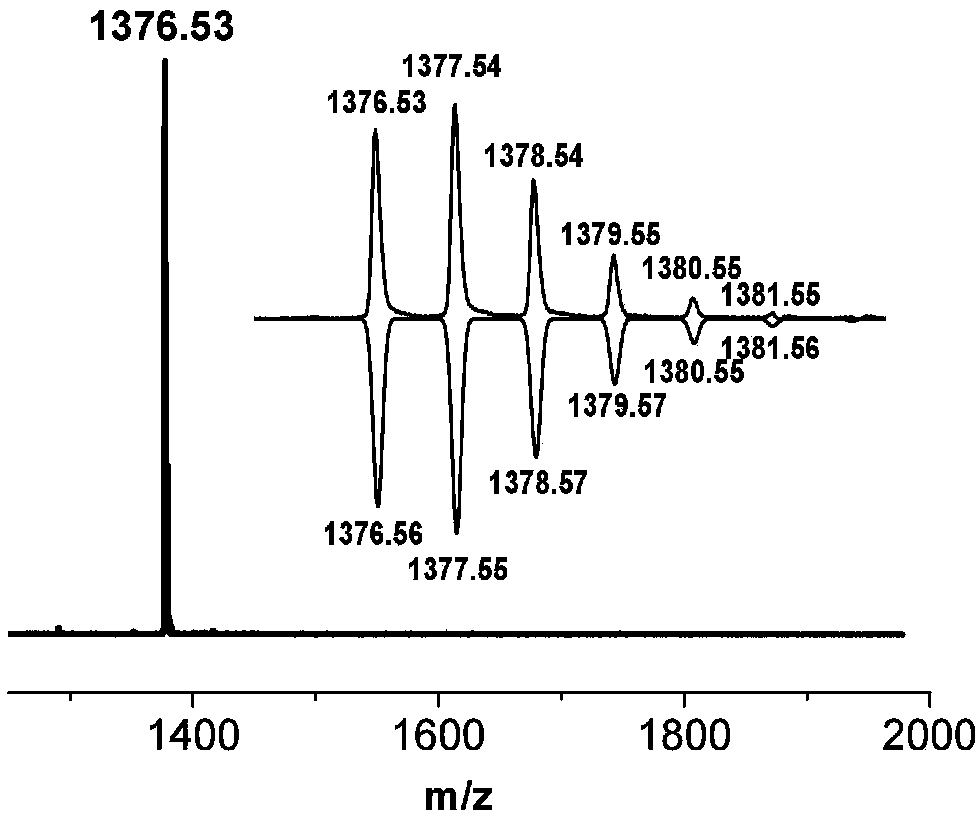
[0047] Add boron trifluoride ether (1ml) and 400ml 1,2-dichloroethane into a 1000ml reaction flask, stir evenly, add 1a (0.300g, 0.519mmol, 1equiv) into a container containing 100ml 1,2-dichloroethane In the constant pressure dropping funnel of ethyl chloride, drop it into the reaction bottle at a rate of one drop per second. After the dropwise addition, react for 5-10 hours. After the reaction is complete, add water to quench the reaction. Extracted with dichloromethane, collected the organic phase, dried over anhydrous magnesium sulfate, filtered off the desiccant, and distilled off the solvent under reduced pressure. The crude product was further separated and purified by silica gel chromatography to obtain white solid powder 2a (0.102g, 35.1 %).
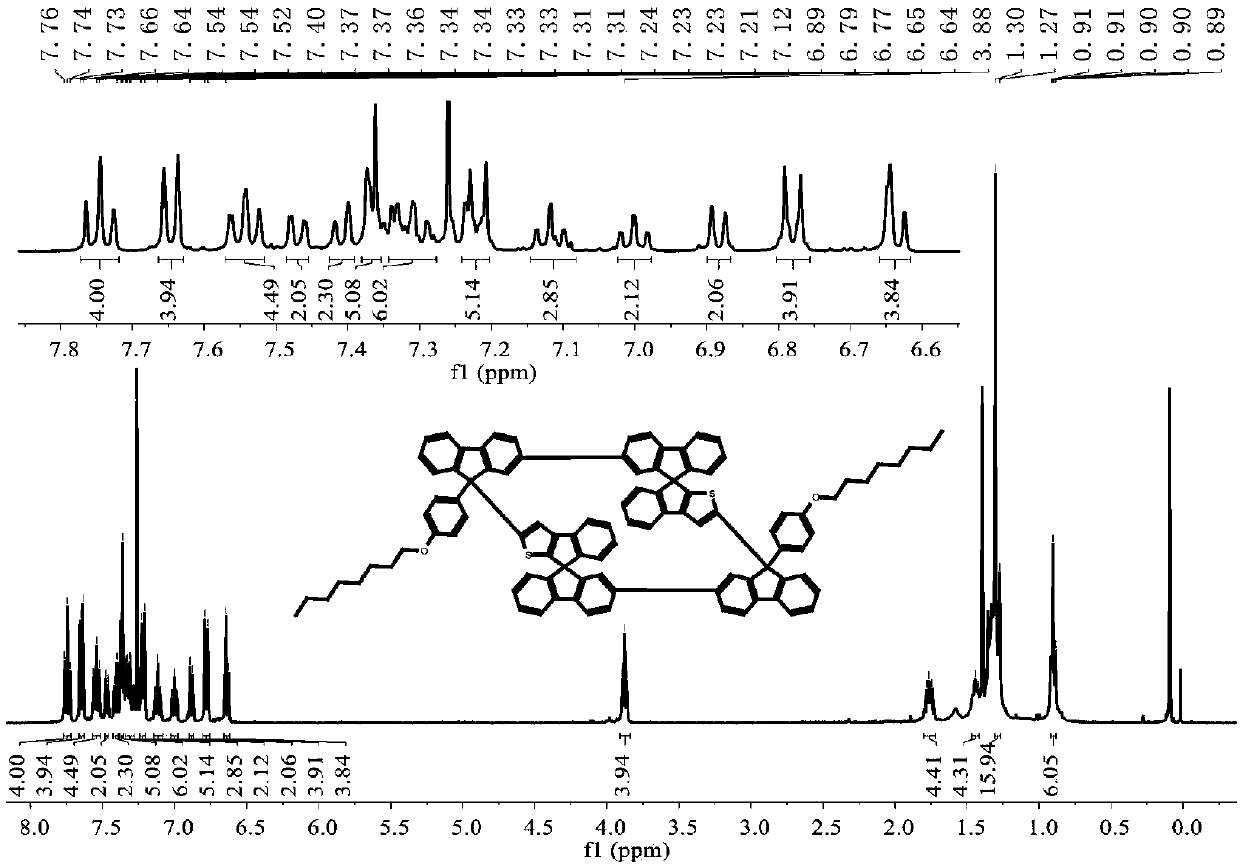
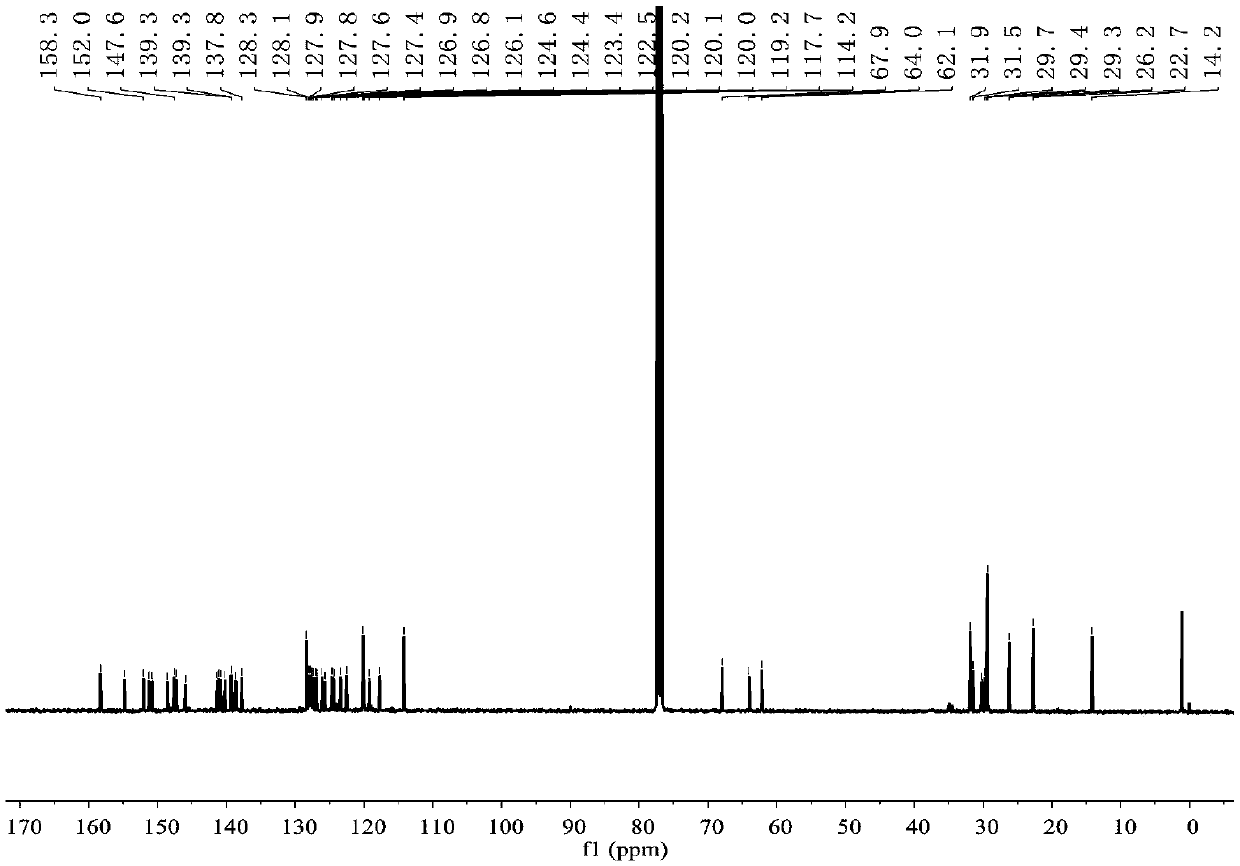
[0048] 1 H NMR (400MHz, CD 2 Cl 2 )δ7.81–7.78(d,J=8.0Hz,4H),7.70–7.69(d,J=8.0Hz,4H),7.58–7.56(d,J=7.6Hz,2H),7.55–7.53(d ,J=7.6Hz,2H),7.50–7.47(m,3H),7.46–7.44(d,...
example 2
[0049] Example 2: Preparation of Rhombus Cell-2b
[0050]
[0051] Add boron trifluoride ether (2ml) and 900ml 1,2-dichloroethane into a 1000ml reaction flask, stir evenly, add 1b (0.600g, 1.01mmol, 1equiv) into a container containing 100ml 1,2-dichloroethane In the constant pressure dropping funnel of ethyl chloride, drop it into the reaction bottle at a rate of one drop per second. After the dropwise addition, react for 5-10 hours. After the reaction is complete, add water to quench the reaction. Extracted with dichloromethane, collected the organic phase, dried over anhydrous magnesium sulfate, filtered off the desiccant, and distilled off the solvent under reduced pressure. The crude product was further separated and purified by silica gel chromatography to obtain white solid powder 2b (0.291g, 50.0 %).
[0052] 1 H NMR (400MHz, CDCl 3 )δ7.74–7.70(t,J=7.8Hz,2H),7.65–7.59(m,4H),7.54–7.45(m,8H),7.41–7.37(m,4H),7.33–7.29(m, 6H),7.21–7.17(m,8H),7.11–7.05(m,6H),6.99–6.95...
example 3
[0053] Example 3: Preparation of Rhombus Cell-2c
[0054]
[0055] Add boron trifluoride ether (2ml) and 900ml 1,2-dichloroethane into a 1000ml reaction flask, stir evenly, add 1c (0.600g, 0.986mmol, 1equiv) into a container containing 100ml 1,2-dichloroethane In the constant pressure dropping funnel of ethyl chloride, drop it into the reaction bottle at a rate of one drop per second. After the dropwise addition, react for 5-10 hours. After the reaction is complete, add water to quench the reaction. Extract with dichloromethane, collect the organic phase, dry over anhydrous magnesium sulfate, filter off the desiccant, and distill off the solvent under reduced pressure. The crude product is further separated and purified by silica gel chromatography to obtain white solid powder 2c (0.289g, 49.6 %).
[0056] 1 H NMR (400MHz, CDCl 3 )δ7.66–7.59(m,6H),7.54–7.48(d,J=9.2,6.8Hz,8H),7.44–7.41(m,4H),7.32–7.29(d,J=10.4Hz,4H) ,7.24–7.18(d,J=8.0Hz,6H),7.11–7.09(d,J=7.6Hz,2H),7.07–7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com