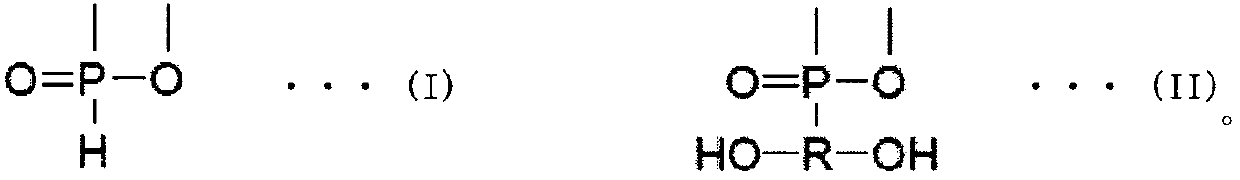
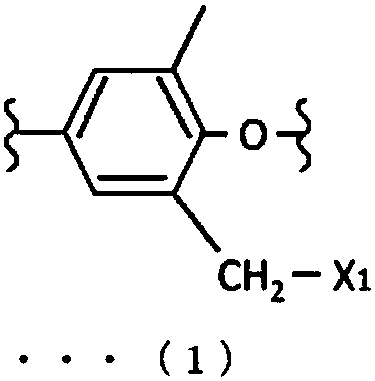
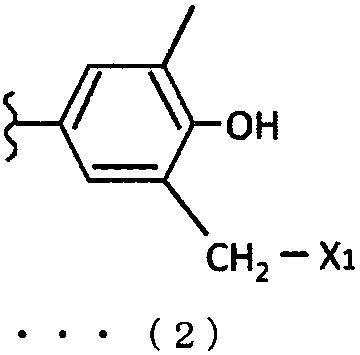
Laminated molded article
A technology of laminated molding and molded products, which is applied in the direction of coating, layered products, metal layered products, etc., can solve the problem of no disclosure of the adhesion between the metal vapor deposition layer and the resin composition, and achieve excellent adhesion , the effect of high surface reflectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、8、14、15
[0284] (i-1) 70 parts by mass and (ii) 0.7 parts by mass were mixed with a drum mixer, and the powder mixture was extruded from a TEM58SS twin-screw extruder (manufactured by Toshiba Machinery Co., Ltd., number of barrels 13, screw diameter 58 mm, L / D=53; screw mode with kneading disc L: 2, kneading disc R: 14 and kneading disc N: 2) the most upstream part (top feed port) supply, at the barrel temperature Melt kneading was carried out under the conditions of 300° C., screw rotation speed of 400 rpm, extrusion speed of 400 kg / hr, and exhaust port vacuum of 7.998 kPa (60 Torr) to obtain pellets. The pellets were dissolved in chloroform, followed by reprecipitation with methanol to extract polyphenylene ether components. Thereafter, it was vacuum-dried at 60° C. for 4 hours to obtain a polyphenylene ether powder.
[0285] The resulting polyphenylene ether powder can be used 31 P-NMR (single plus method) and 1 H-NMR was identified, and the addition amount of the reactive compou...
Embodiment 2、10
[0332] A mixture of (i-2) 70 parts by mass and (ii) 0.7 parts by mass was melt-kneaded in the same manner as in Example 1 to obtain pellets. The obtained pellets were made into polyphenylene ether powder, and the addition amount of the reactive compound was quantified in the same manner as in Example 1. As a result, it was confirmed that the chemical formula (9) per 100 monomers in the polyphenylene ether chain , and (10) have a total of 0.26 structural units.
[0333] Furthermore, the amount of addition on the terminal hydroxyl group can be utilized 13 C-NMR, using the peak integral value [A] of 146.4ppm (the carbon adjacent to the oxygen atom of the ether bond (which is formed by adding a reactive compound to the OH group), 145.4ppm (the carbon adjacent to the OH group) The integral value [B] of the adjacent carbon) was obtained from the above formula (1), and it was confirmed that 0.04 structural units of the chemical formula (11) were included in every 100 monomer units c...
Embodiment 3、4、5、6、7、12、18
[0337] A mixture of (i-1) 40 parts by mass, (i-2) 30 parts by mass, and (ii) 0.7 parts by mass was melt-kneaded in the same manner as in Example 1 to obtain pellets. The obtained pellets were made into polyphenylene ether powder, and the addition amount of the reactive compound was quantified in the same manner as in Example 1. As a result, it was confirmed that the chemical formula (9) per 100 monomers in the polyphenylene ether chain , and (10) have a total of 0.28 structural units.
[0338] Furthermore, the amount of addition on the terminal hydroxyl group can be utilized 13 C-NMR, using the peak integral value [A] of 146.4ppm (the carbon adjacent to the oxygen atom of the ether bond (which is formed by adding a reactive compound to the OH group), 145.4ppm (the carbon adjacent to the OH group) The integral value [B] of the adjacent carbon) was obtained from the above formula (1), and it was confirmed that 0.03 structural units of the chemical formula (11) were included in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com