A kind of method for preparing sodium ethylenediamine ethanesulfonate
A technology of sodium ethylenediaminoethanesulfonate and sodium taurine, which is applied in the direction of sulfonic acid preparation, sulfonate preparation, organic chemistry, etc., and can solve the problem that sulfonic acid-type hydrophilic chain extenders do not have cost advantages and impurities Difficult separation, low product purity, etc., to achieve good industrialization prospects, mild reaction conditions, and high product selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
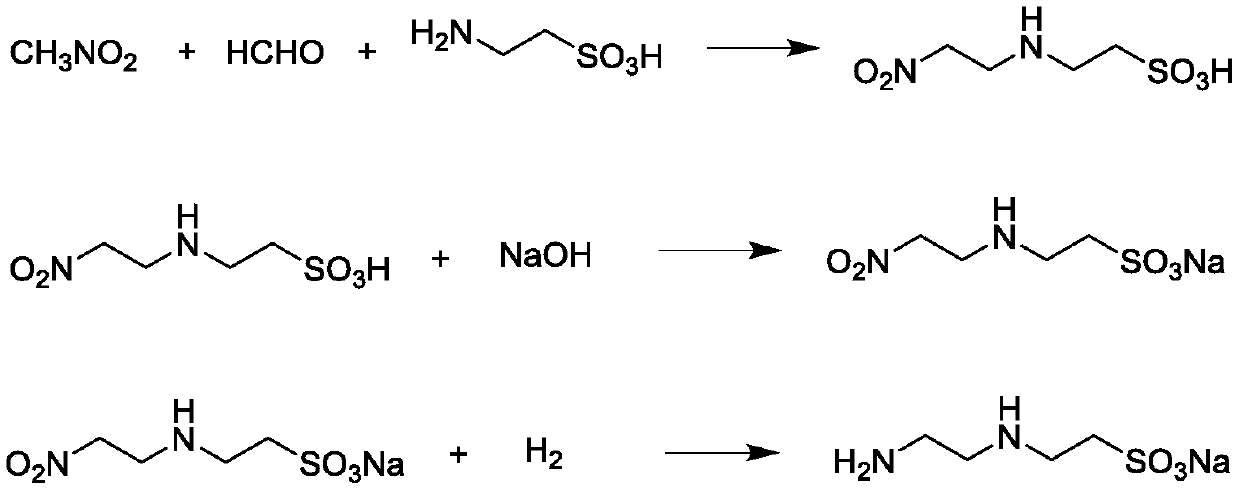
[0039] (1) Add 125 g of taurine, 118 g of 37wt% aqueous formaldehyde solution and 650 g of ethanol to a three-necked flask, stir at 25°C for 10 minutes, drop 68 g of nitromethane into the three-necked flask within 30 minutes, and heat to After stirring for 20 hours at 30°C, the reaction solution was distilled under reduced pressure to remove light components. The solid was washed with 300 grams of water and vacuum dried at 50°C for 4 hours to obtain a light yellow solid N-(2-nitroethyl)taurine 194.4 g, yield 98.0%, selectivity 99.8%. IR(KBr): ν=3340cm -1 (N-H), ν=1550cm -1 (NO 2 ), ν=1200cm -1 (SO 3 ); 1 H NMR (400MHz, DMSO-D6), δ = 2.76 (t, 2H), δ = 3.01 (t, 2H), δ = 3.14 (t, 2H), δ = 4.69 (t, 2H).
[0040] (2) Under stirring, slowly add 99 g of N-(2-nitroethyl) taurine to 66.7 g of 30wt% sodium hydroxide aqueous solution, control the temperature at 30°C, and continue stirring for 10 minutes after the solid is added completely; The water was removed by pressure distillation to ...
Embodiment 2
[0044] (1) Add 125 g of taurine, 35 g of paraformaldehyde and 2500 g of methanol to a three-necked flask, stir at 25°C for 20 minutes, drop 88 g of nitromethane into the three-necked flask within 30 minutes, and heat to 70 After stirring for 4 hours at ℃, the reaction solution was distilled under reduced pressure to remove light components. The solid was washed with 260 grams of water and dried under vacuum at 50 ℃ for 4 hours to obtain light yellow solid N-(2-nitroethyl)taurine 195.3 G, the yield is 98.5%, and the selectivity is 99.9%.
[0045] (2) Under stirring, slowly add 99 g of N-(2-nitroethyl) taurine to 220 g of 20wt% sodium bicarbonate aqueous solution, control the temperature at 40°C, and continue stirring for 15 minutes after the solid is added completely; The water was removed by pressure distillation to obtain 110 g of sodium N-(2-nitroethyl) taurate.
[0046] (3) Put 110 grams of sodium N-(2-nitroethyl) taurate, 7000 grams of ethanol, and 1 gram of Raney nickel catal...
Embodiment 3
[0049] (1) Add 125 g of taurine, 95 g of 37wt% formaldehyde aqueous solution and 1500 g of dimethyl sulfoxide to a three-necked flask, stir at 25°C for 10 minutes, and drop 76 g of nitromethane into the three-necked flask within 30 minutes After heating to 55°C and stirring for 6 hours, the reaction solution was distilled under reduced pressure to remove light components. The solid was washed with 300 grams of water and vacuum dried at 50°C for 4 hours to obtain a light yellow solid N-(2-nitroethyl ) Taurine is 194.9 g, the yield is 98.2%, and the selectivity is 99.8%.
[0050] (2) Under stirring, slowly add 99 grams of N-(2-nitroethyl) taurine to 200 grams of 10wt% aqueous sodium hydroxide solution, control the temperature at 35°C, and continue stirring for 10 minutes after the solids are completely added; The water was removed by pressure distillation to obtain 110 g of sodium N-(2-nitroethyl) taurate.
[0051] (3) Put 110 grams of N-(2-nitroethyl) sodium taurate, 3000 grams of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com