Preparation method for naphthalene alkylation catalyst
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Take 50g of water glass (SiO 2 The content is 27.20wt%, the modulus is 3.2), 0.67g aluminum sulfate, 96wt% sodium hydroxide 1.0g, deionized water 50g, 30wt% tetraethylammonium hydroxide aqueous solution 50g, mixed into a gel, with Adjust the pH to 13 with 10% sodium hydroxide aqueous solution and let it stand for 6 hours. Then it was crystallized at 170°C for 72 hours.
[0032] (2) Take the milky white slurry obtained in the previous step and wash it with deionized water 5 times, and then vacuum dry for 12 hours.
[0033] (3) Take the solid obtained in the previous step and calcinate in a muffle furnace at 550°C for 6 hours.
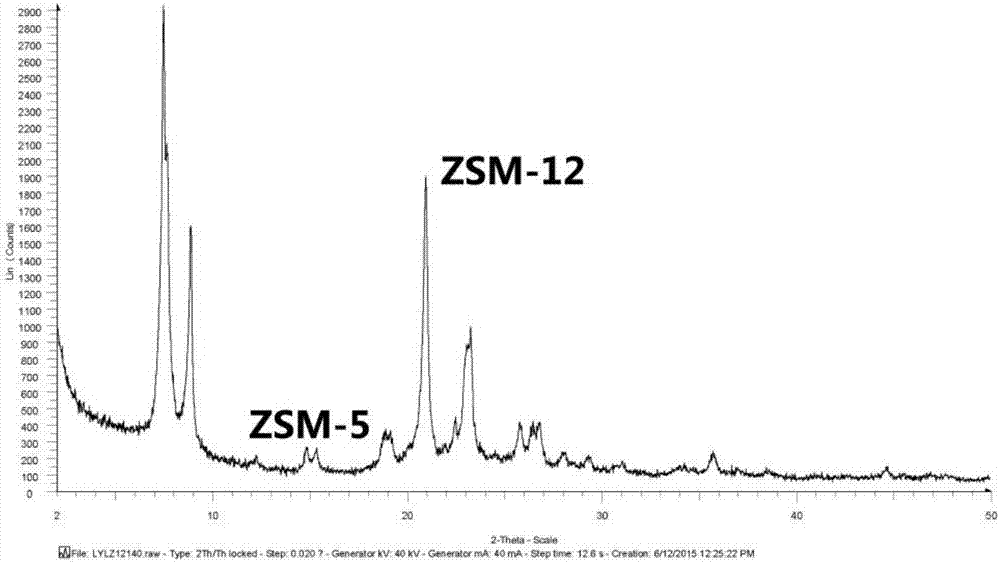
[0034] The original mixed crystal powder obtained in the previous step is numbered as A1, and it is detected as a mixture of ZSM-5 and ZSM-12 by XRD powder diffraction. The ratio of ZSM-5 to ZSM-12 is 1:1.
[0035] (4) Take 10.3g of the powder obtained in the previous step and use 1mol / L of NH 4 NO 3 Exchange the 200ml solution three times at room t...
Embodiment 2
[0041] (1) 600 grams of 40% silica sol, 10.1 grams of sodium aluminate (42% alumina content), 41.6 grams of 30% by weight tetraethylammonium hydroxide (TEAOH), 168.0 grams of tetraethylammonium bromide (TEABr), Mix 16.0 grams of sodium hydroxide with 925.1 grams of water, stir evenly at room temperature, and let stand for 6 hours. Then it was crystallized at 200°C for 48 hours.
[0042] (2) Take the milky white slurry obtained in the previous step and wash it with deionized water 5 times, and then vacuum dry for 12 hours.
[0043] (3) Take the solid obtained in the previous step and calcinate in a muffle furnace at 550°C for 6 hours.
[0044] The mixed crystal powder obtained in the previous step is numbered as A2, and it is detected as a mixture of ZSM-5 and ZSM-12 by XRD powder diffraction. The ratio of ZSM-5 to ZSM-12 is 1:1.
[0045] (4) Take 200g of the powder obtained in the previous step and use 1mol / L of NH 4 NO 3 The 2000ml solution was exchanged three times at 80°C for 8 h...
Embodiment 3
[0051] (1) Take 3.5g of aluminum tert-butoxide (wt97%), 160g of 30wt% tetraethylammonium hydroxide, 8g of sodium hydroxide and mix, then add 160g of TEOS (wt98%) and 140g of deionized water, at room temperature Stir well and let stand for 4 hours. Then it was crystallized at 200°C for 48 hours.
[0052] (2) Take the milky white slurry obtained in the previous step and wash it with deionized water 5 times, and then vacuum dry for 12 hours.
[0053] (3) Take the solid obtained in the previous step and calcinate in a muffle furnace at 550°C for 6 hours.
[0054] The original mixed crystal powder obtained in the previous step was numbered as A3, and it was detected as a mixture of ZSM-5 and ZSM-12 by XRD powder diffraction. The ratio of ZSM-5 to ZSM-12 is 1:4.
[0055] (4) Take 35g of the powder obtained in the previous step and use 2mol / L of NH 4 Exchange 500 ml of Cl solution three times at 80°C for 8 hours each time.
[0056] (5) Take the solid obtained in the previous step, dry, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com