PCV2 (porcine circovirus 2) recombinant baculovirus-like particle subunit vaccine and preparation method
A recombinant baculovirus and subunit vaccine technology, applied in the field of bioengineering, can solve problems such as lack of highly immunogenic PCV2 vaccine, and achieve the effect of reducing vaccine impurities, high content, and mild animal reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
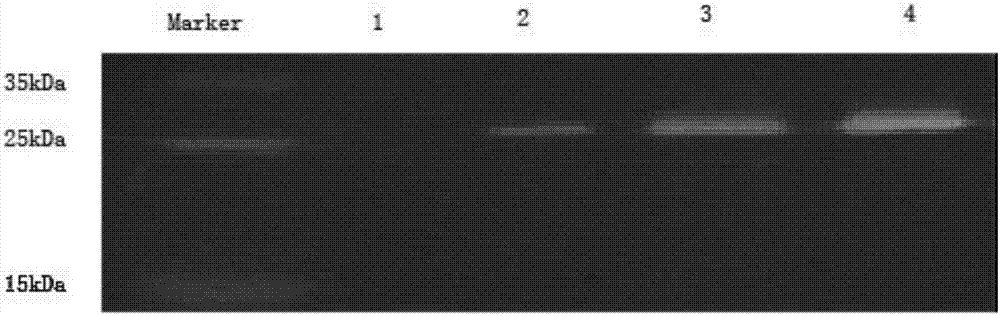
Image
Examples
Embodiment 1
[0038] The acquisition of embodiment 1PCV2ORF2 recombinant baculovirus
[0039] The plasmid pUC57-PCV2 (synthesized by Sangon Bioengineering (Shanghai) Co., Ltd.) containing the codon PCV2 recombinant baculovirus ORF2 nucleotide sequence was artificially synthesized. The nucleotide sequence of PCV2 ORF2 is GenBank AIT11738.1 sequence. According to the multiple cloning site sequence of the shuttle vector pAcGP67-A, EcoR1 and BglII restriction sites were added to the 5' and 3' ends of the ORF2 sequence, respectively. The PCV2 recombinant baculovirus ORF2 gene synthesized in the present invention has a full length of 713bp, and its specific sequence is shown in SEQ ID NO.1.
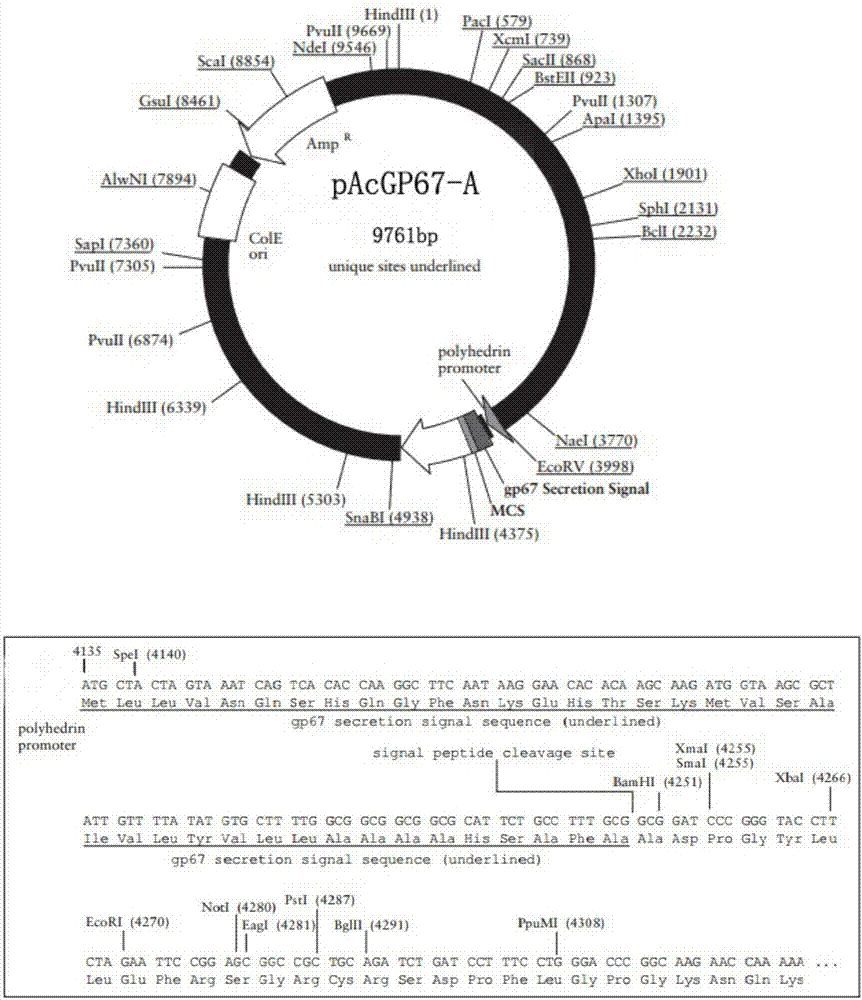
[0040] The recombinant plasmid pUC57-PCV2 was digested with EcoR1 / BglII, and the target fragment was purified and recovered. The recovered target fragment was cloned into the shuttle vector pAcGP67-A (BD BiosciencesPharmingen, San Diego, CA, such as figure 1 shown), the recombinant shuttle vector pAcGP67-...
Embodiment 2
[0044] Example 2 Preparation of PCV2 recombinant baculovirus-like particles
[0045] 1. Cell preparation
[0046] Take the frozen Sf9 working cell bank cells and disperse the cells to 75cm 2 In the cell flask, add EX-CELL TM 420 serum-free medium. Cultivate at 27°C for 24 hours, transfer the cells to the culture flask at a dispersion ratio of 1:5, add new serum-free medium, continue culturing at 27°C, and collect the cells. Passage in the same way, and expand the culture until the required number of cells is expanded. to 4×10 5 The cells / ml cell density is connected to the cell bioreactor for full suspension serum-free culture, and cultured at 27°C until the cell density is 2×10 6 cells / ml, it is used for poisoning.
[0047] 2. Virus inoculation, cultivation and harvesting
[0048] Inoculate the Sf9 working bank cells with a density of 2×106 cells / ml in the bioreactor with the virus seed for production at an inoculation amount of MOI=1, and culture the virus in full sus...
Embodiment 3
[0051] Example 3 Preparation of PCV2 Recombinant Baculovirus-like Particle Subunit Vaccine
[0052] 1. Obtaining the virus stock solution
[0053] The Sf9 working bank cells were cultured in full suspension serum-free, when the cell density reached 1.5×10 6 The cells / ml were infected with the recombinant baculovirus, and the virus inoculation volume was MOI=2. Continue the full suspension serum-free culture for 6 days, and directly harvest the cell culture medium, which is the virus stock solution.
[0054] 2. Filtration of virus liquid
[0055] The harvested cell culture supernatant was filtered and sterilized through a 1.0 μm membrane filter system and a 0.22 μm membrane filter system in sequence, and the filtrate was stored at 2-8°C.
[0056] 3. Virus inactivation
[0057] In order to ensure the safety of the PCV2 recombinant baculovirus-like particle subunit vaccine, we inactivated the recombinant baculovirus in the virus fluid. Diethyleneimine BEI was used as inactiv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com