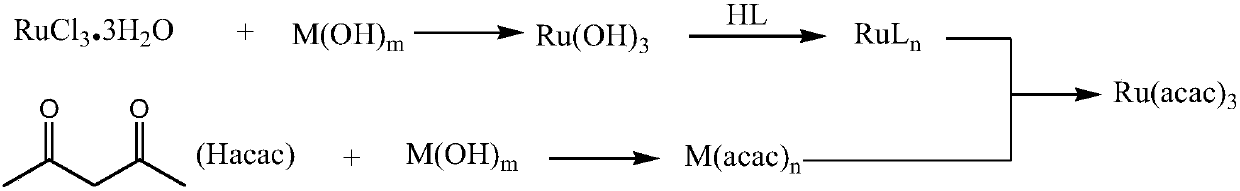
Method for synthesizing ruthenium acetylacetonate (III)
A technology of ruthenium acetylacetonate and a synthesis method, applied in chemical instruments and methods, ruthenium organic compounds, platinum group organic compounds, etc., can solve the problems such as unfavorable acquisition of reaction raw materials, unfavorable amplification of microwave reaction, etc., and achieves reduced content and high yield. rate, the effect of low chloride ion content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Add 10.5g (40mmol) ruthenium trichloride hydrate to 200mL water, stir and dissolve at room temperature, add 30mL of 4mol / L sodium hydroxide solution, a large amount of black precipitate is formed, continue to stir for 1 hour, filter, wash with deionized water, wash with 0.1mol / L silver nitrate solution to measure the content of chloride ions in the washing solution until there is no white turbidity. Transfer the black precipitate to a beaker, add 40mL of 3mol / L perchloric acid solution, heat to 50°C, stir and dissolve for later use.
[0035] Add 12.0 g (120 mmol) of acetylacetone to 60 mL of 2 mol / L sodium hydroxide solution to react to obtain a sodium acetylacetonate solution. Slowly add the above-mentioned standby ruthenium salt solution to the acetylacetonate solution while stirring, and complete the addition After heating at 90°C for 4 hours, a dark red ruthenium(III) acetylacetonate precipitate was formed, which was extracted with 200mLx3 dichloromethane, the orga...
Embodiment 2
[0037] Replace 3mol / L perchloric acid solution with 2mol / L hexafluorophosphoric acid solution, the addition amount is 60mL, the reaction heating temperature of ruthenium salt and acetylacetonate is 100°C, the feeding amount of other reactants and experimental conditions are the same as in Example 1, and the product yield rate of 85%.
Embodiment 3
[0039] Replace 3mol / L perchloric acid solution with 2mol / L tetrafluoroboric acid solution, the addition amount is 60mL, the reaction heating temperature of ruthenium salt and acetylacetonate is 80°C, the feeding amount of other reactants and experimental conditions are the same as in Example 1, and the product yield The rate is 83%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com