Preparation method of non-noble metal nitrogen-doped porous carbon electrocatalyst
A non-precious metal, electrocatalyst technology, applied in chemical instruments and methods, physical/chemical process catalysts, circuits, etc., can solve the problems of unfavorable mass transfer, low oxygen reduction catalytic performance, low volume specific activity, etc., to achieve preparation The process is simple, environmentally friendly and safe, it is convenient for large-scale production, and the effect of simplifying the preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1. Take 3.534g of melamine in a single-necked flask, add 113mL of ethylene glycol, stir and heat to dissolve at 80°C, then add 0.3534g of MOF-5, that is, the mass ratio of nitrogen source to MOF is 10:1; react for 4 hours The solvent was then evaporated to dryness to obtain a white powder.
[0028] 2. Pyrolyze the above-mentioned white powder in a quartz boat of a tube furnace, pass in argon gas with a flow rate of 80mL / min, heat up at a rate of 5°C per minute, keep at 900°C for 3 hours, and then cool down naturally to obtain MOF-10 Melamine-900 catalyst.
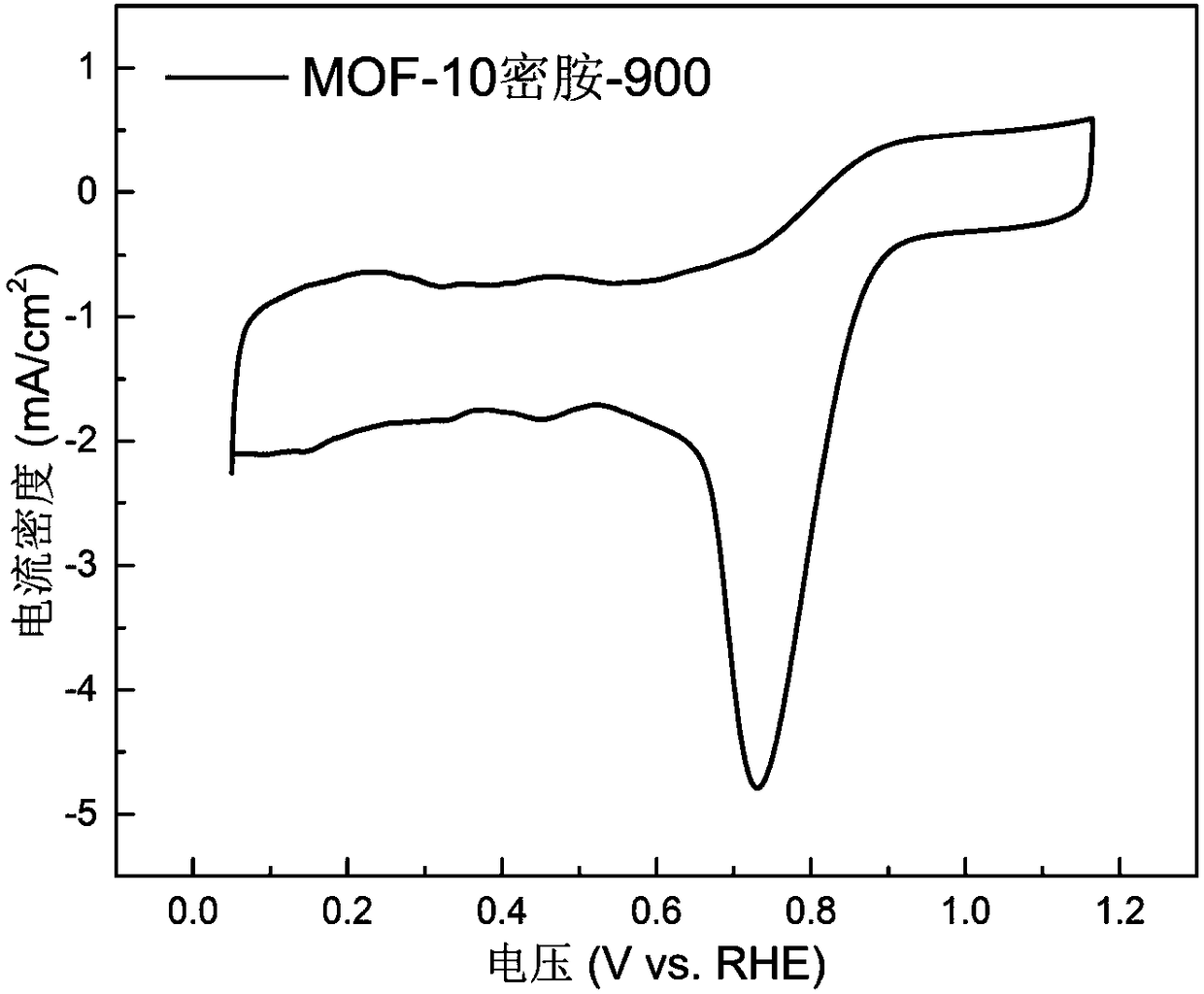
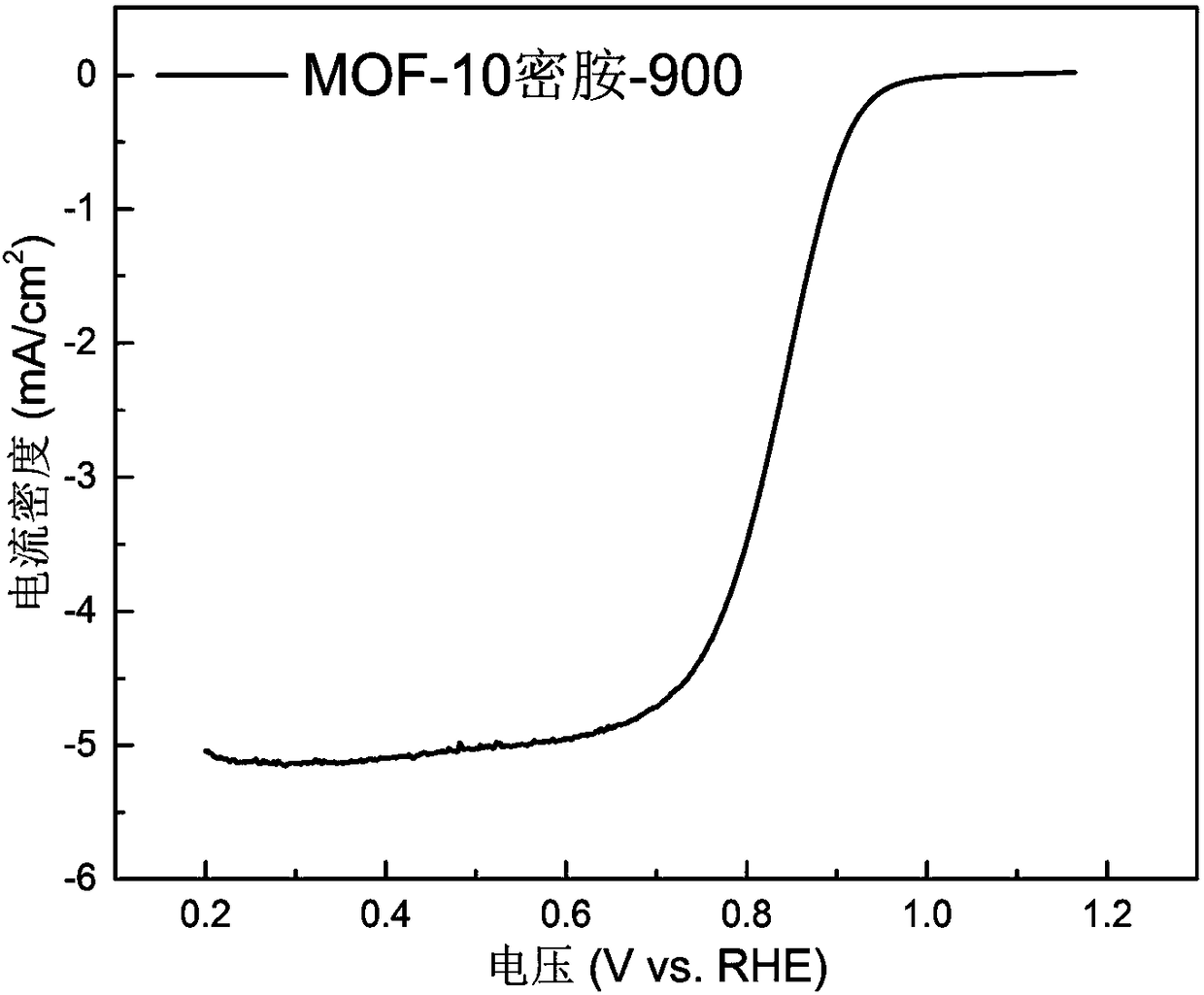
[0029] figure 1 and figure 2 The cyclic voltammetry curves and oxygen reduction polarization curves of the MOF-10 melamine-900 catalyst prepared in Example 1 were tested by rotating disk electrode (RDE).
Embodiment 2
[0031] 1. Take 3.534g of melamine in a single-necked flask, add 113mL of ethylene glycol, stir and heat to dissolve at 80°C, then add 0.3534g of MIL-101(Fe), that is, the mass ratio of nitrogen source to MOF is 10:1; After reacting for 4 hours, the solvent was evaporated to dryness to obtain a light yellow powder.
[0032] 2. Pyrolyze the above-mentioned light yellow powder in a quartz boat of a tube furnace, pass in argon gas with a flow rate of 80mL / min, heat up at a rate of 5°C per minute, keep at 900°C for 3 hours and then cool down naturally to obtain MIL- 10 Melamine-900 catalyst.
Embodiment 3
[0034] 1. Take 3.534g of dicyandiamide in a single-necked flask, add 113mL of ethanol, stir and heat to dissolve at 80°C, then add 0.6311g of ZIF-8, that is, the mass ratio of nitrogen source to MOF is 5.6:1; after 4 hours of reaction The solvent was evaporated to dryness to obtain a white powder.
[0035] 2. Pyrolyze the above-mentioned white powder in a quartz boat of a tube furnace, pass in argon gas at a flow rate of 80mL / min, heat up at a rate of 5°C per minute, keep at 800°C for 2 hours, and then cool down naturally to obtain ZIF-5.6 DCD-800 catalyst.
[0036] image 3 The SEM figure of the ZIF-5.6DCD-800 catalyst prepared for embodiment three; Figure 4 It is the XRD pattern of the corresponding ZIF-5.6DCD-800 catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com