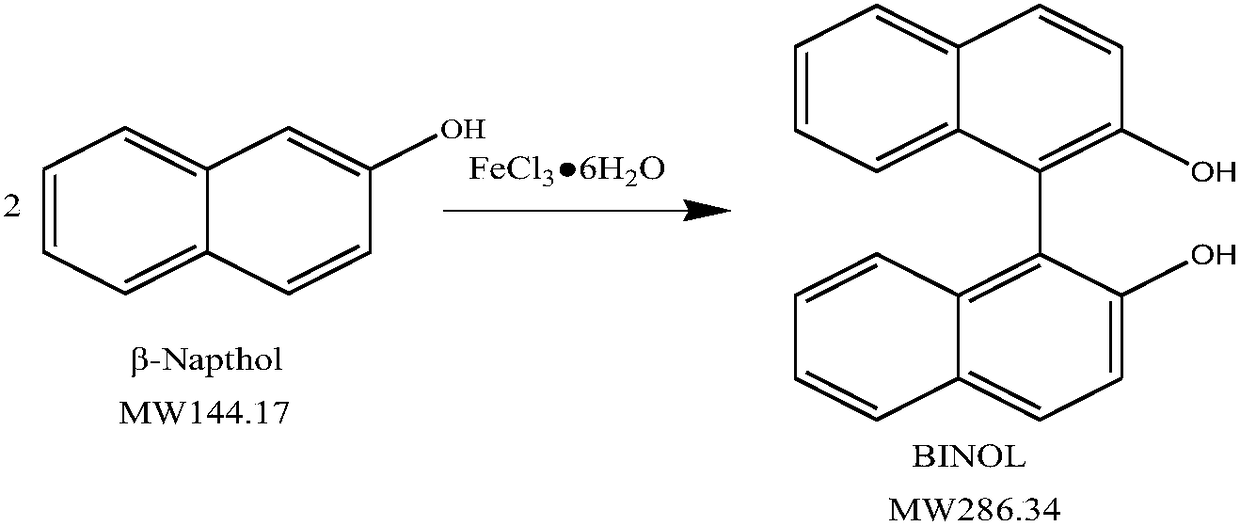
Method for preparing electronic 2,2'-binaphthol
An electronic-grade, binaphthol technology, applied in the field of compound preparation, can solve problems such as product yield, quality and cost are difficult to meet, product purity is difficult to reach electronic level, unfavorable to occupational health and labor protection, etc., to achieve production The effect of good environment, easy operation and low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A preparation method of electronic grade 2,2'-binaphthol, the specific steps are as follows:
[0039] In a 250mL four-necked flask, equipped with a thermometer, an electric stirrer, and a reflux condenser, dissolve 7.2g of 2-naphthol and 0.72g of a phase transfer catalyst (TBAB) in 9.5g of methanol, and stir Heat up to 70°C to dissolve, then add 130.0g of 17.6% ferric chloride aqueous solution and 0.96g of 30% hydrogen peroxide in batches under heat preservation conditions, and heat at 70°C for 1 hour.
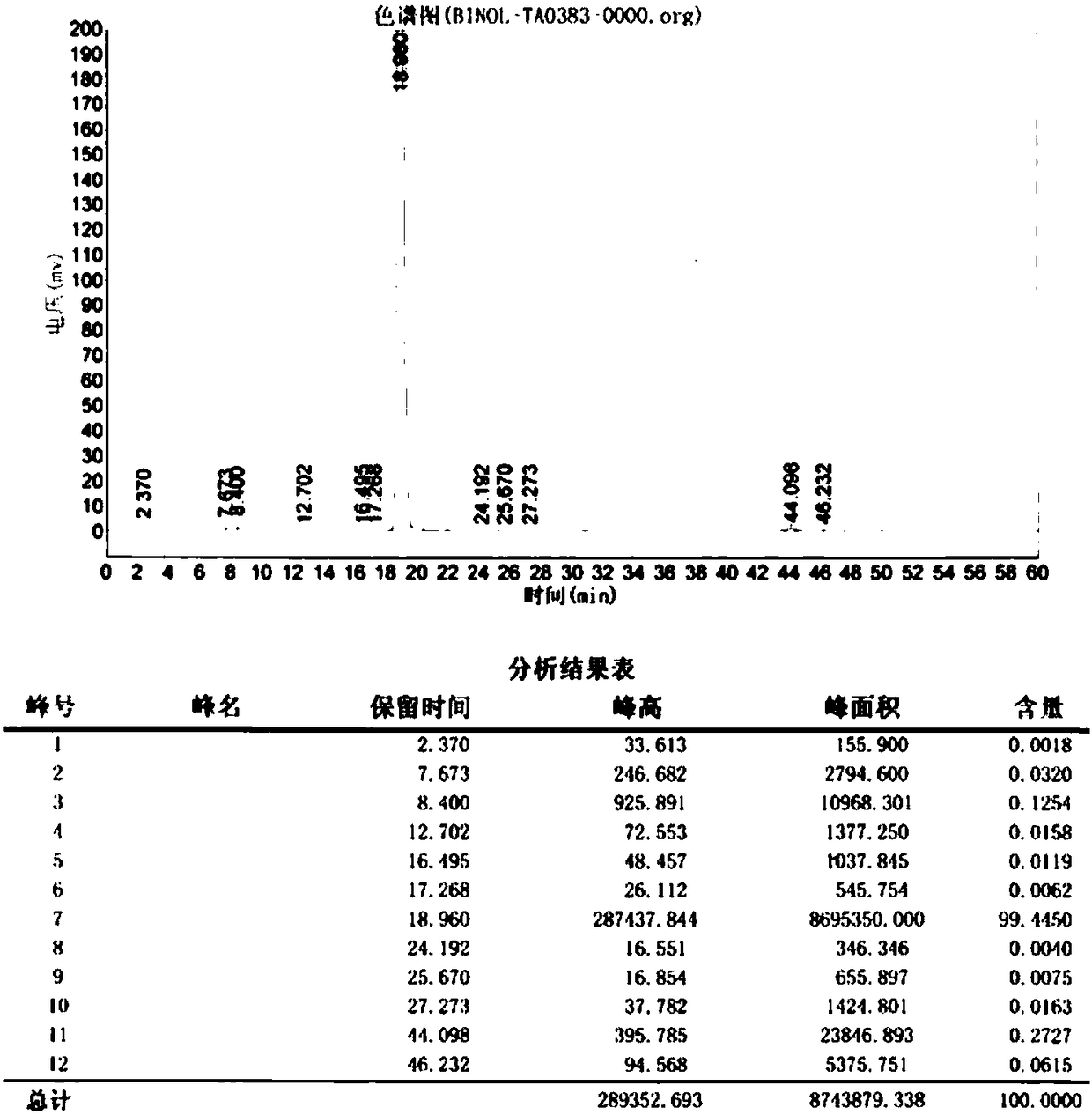
[0040] The reaction progress of the mixed solution is monitored, and when the high-performance liquid chromatography monitors that the 2-naphthol content is less than or equal to 3.0%, the reaction is stopped. Then 8.0 g of 1,2-dichloroethane was added, and the reaction was continued to stir at 60°C for 30 minutes, cooled to crystallize, filtered, washed, and dried to obtain 6.58 g of 2,2'-binaphthol with a yield of 92.0%.
[0041] Product YI value is measured as 3.5 b...
Embodiment 2~4
[0043] Examples 2-4 all basically adopt the following preparation method of electronic grade 2,2'-binaphthol, the specific steps are as follows, the difference is that the phase transfer catalysts added in Examples 2-4 are different, and the dosage is also different. As shown in Table 1:
[0044] In a 250mL four-neck flask equipped with a thermometer, an electric stirrer, and a reflux condenser, dissolve 7.2g of 2-naphthol and a phase transfer catalyst in 9.5g of methanol, heat up to 70°C under stirring to dissolve, and then Add 76.70g mass concentration in batches of 17.6% ferric chloride aqueous solution and 30% hydrogen peroxide (the amount of hydrogen peroxide in Examples 2-4 is different, see Table 1 respectively;) 70°C insulation reaction for 1 hour.
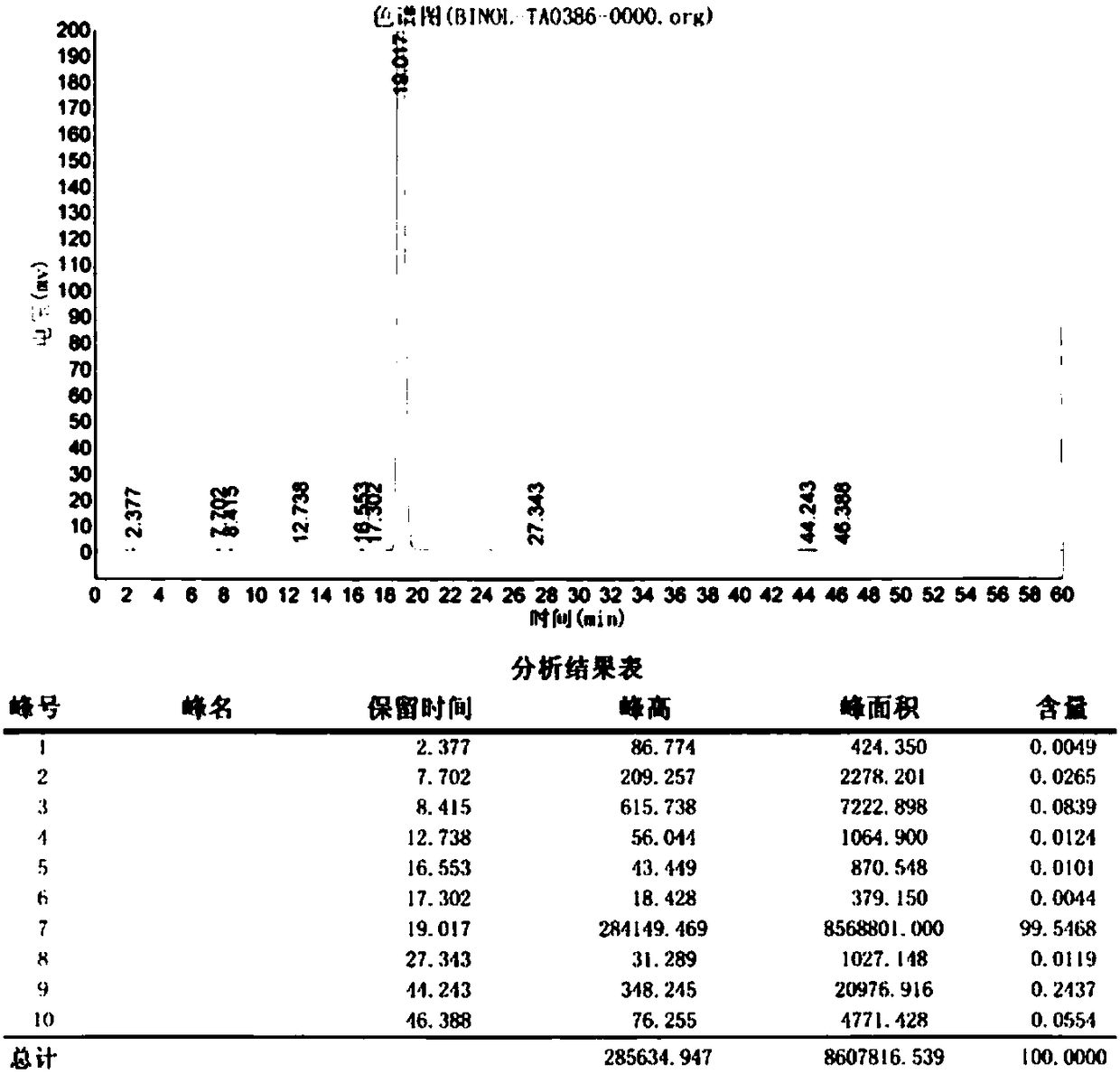
[0045] The reaction progress of the mixed solution is monitored, and when the high-performance liquid chromatography monitors that the 2-naphthol content is less than or equal to 3.0%, the reaction is stopped. Then add 8....
Embodiment 5
[0049] A preparation method of electronic grade 2,2'-binaphthol, the specific steps are as follows:
[0050] In a 250mL four-necked bottle, equipped with a thermometer, an electric stirrer, and a reflux condenser, add 9.5g of methanol, 14.4g of 2-naphthol, and 0.72g of sodium dodecylbenzenesulfonate, and heat up to 70°C under stirring to dissolve. Then, 114.9 g of ferric chloride aqueous solution with a mass concentration of 23.5% and 2.83 g of hydrogen peroxide with a mass concentration of 30% were added in batches under heat preservation conditions, and the reaction was carried out at 75° C. for 1 hour.
[0051] The reaction progress of the mixed solution is monitored, and when the high-performance liquid chromatography monitors that the 2-naphthol content is less than or equal to 3.0%, the reaction is stopped. Then add 12.0 g of toluene, continue to stir and react at 70°C for 30 min, cool down to crystallize, filter, wash, and dry to obtain 12.87 g of 2,2'-binaphthol with a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com