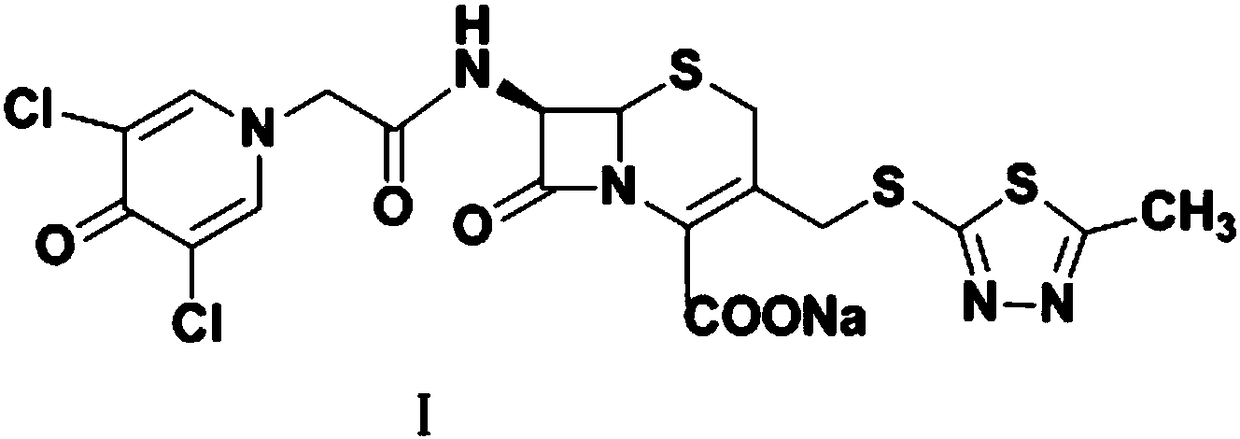
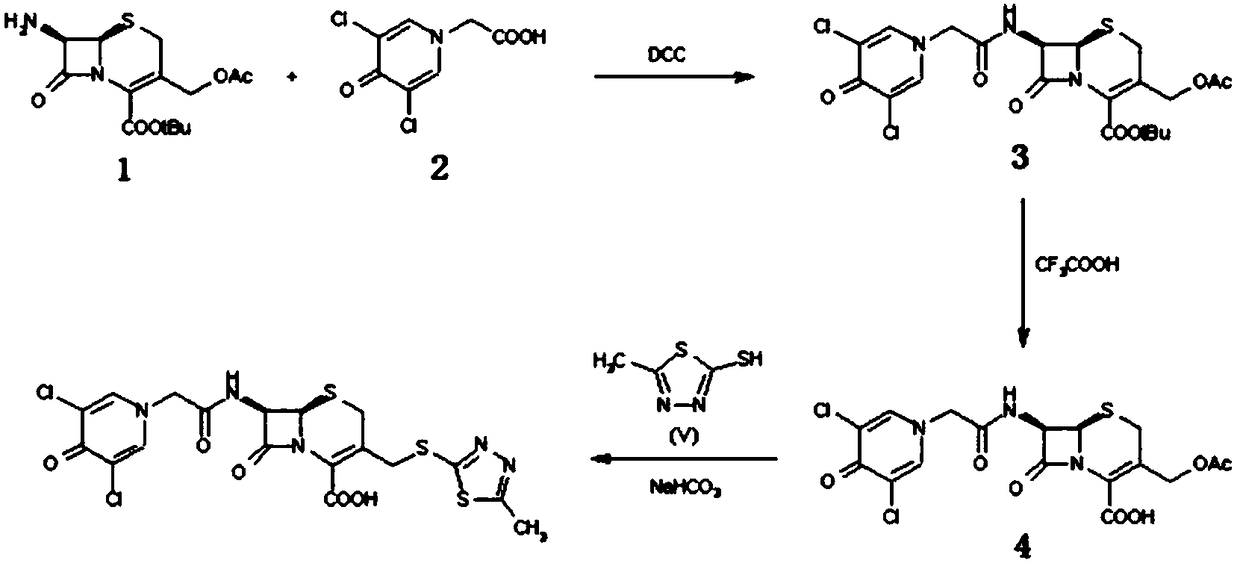
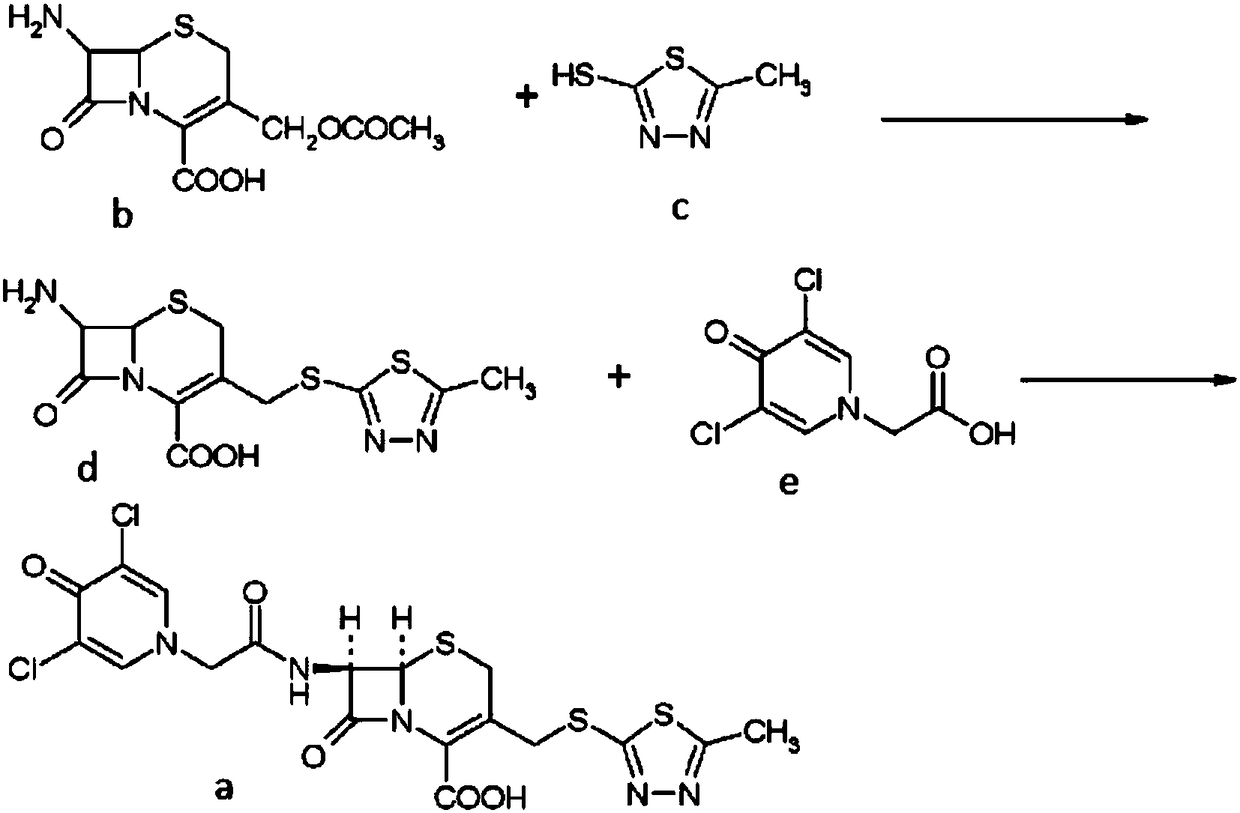
Preparation method of cefazedone sodium compound
The technology of cefoxidone sodium and compound is applied in the field of preparation of anti-infective drug cefoxidone sodium compound, which can solve the problems of many side reactions, lower total product yield, low final product yield and the like, and achieves simple process route, The effect of high total yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation of compound Ⅳ
[0032] Add 27.23g of 7-ACA(II), 13.22g of compound III and 600ml of N,N-dimethylformamide in sequence in the reaction bottle, control the temperature at 40-50°C and stir the reaction for 3h, monitor the completion of the reaction by TLC, then evaporate to remove Solvent, 800ml of ethyl acetate and 800ml of saturated sodium bicarbonate were added to the obtained oil, and the layers were separated. The organic layer was dried over anhydrous sodium sulfate and filtered. After the filtrate was concentrated, it was recrystallized with ether to obtain a solid of 31.87g. The molar yield was 92.4%. , HPLC purity of 99.86%.
Embodiment 2
[0034] Preparation of compound Ⅳ
[0035] Add 27.23g of 7-ACA(II), 13.22g of compound III and 600ml of methylene chloride in sequence in the reaction flask, control the temperature at 40-50°C and stir the reaction for 3h, monitor the completion of the reaction by TLC, and then evaporate the solvent to obtain the oil Add 800ml of ethyl acetate and 800ml of saturated sodium bicarbonate, separate layers, dry the organic layer over anhydrous sodium sulfate, filter, concentrate the filtrate, and recrystallize with ether to obtain 31.34g of solid, with a molar yield of 90.8% and an HPLC purity of 99.78% .
Embodiment 3
[0037] Preparation of compound Ⅳ
[0038] Add 27.23g of 7-ACA(II), 14.54g of compound III and 600ml of N,N-dimethylformamide in sequence in the reaction bottle, control the temperature at 40-50°C and stir the reaction for 3h, monitor the completion of the reaction by TLC, and then remove it by evaporation Solvent, 800ml of ethyl acetate and 800ml of saturated sodium bicarbonate were added to the obtained oil, and the layers were separated. The organic layer was dried over anhydrous sodium sulfate and filtered. After the filtrate was concentrated, it was recrystallized with ether to obtain a solid of 32.29g. The molar yield was 93.6%. , HPLC purity of 99.85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com