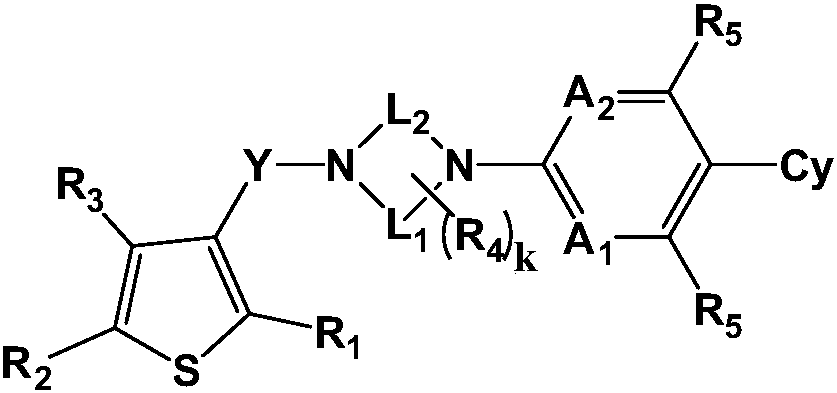
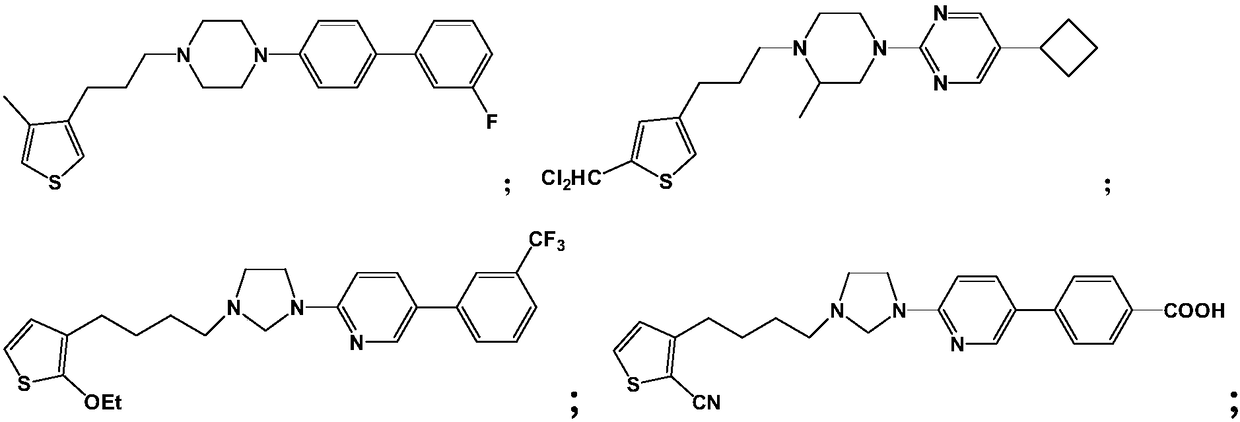
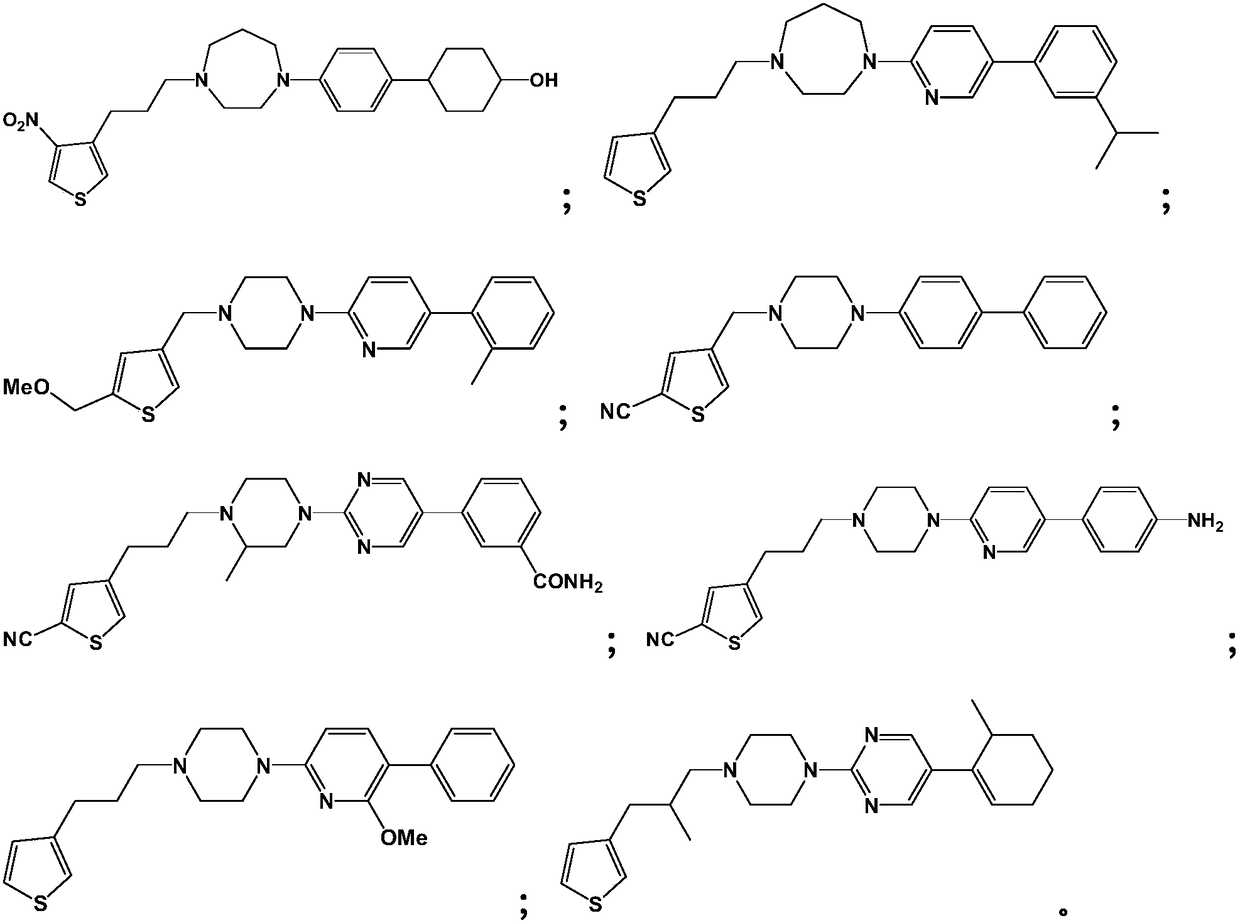
A kind of medicine for preventing and treating diabetic retinopathy and preparation method thereof
A diabetes and drug technology, applied in the direction of drug combination, pharmaceutical formula, urinary system diseases, etc., can solve the problem of prolonging the effective vision of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Example 1: Preparation of 1-(3'-fluorobiphenyl-4-yl)-4-(3-(4-methylthiophen-3-yl)propyl)piperazine (compound 1)
[0072]
[0073] Step 1. Dissolve 10.0 mmol of 3-(4-methylthiophen-3-yl)propan-1-ol in 100 mL of chloroform, and add 3 mL of triethylamine and 12.0 mmol of p-toluenesulfonyl chloride to it in sequence. After stirring the reaction at room temperature for 4 hours, 200 mL of water was added to quench. The organic phase was separated, dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure, and the resulting residue was purified by silica gel column chromatography (cyclohexane / ethyl acetate (v / v)=3 / 1) to obtain 3-(4 -Methylthiophen-3-yl)propyl p-toluenesulfonate 2.54 g (82.1%).
[0074] Under nitrogen protection, 3-(4-methylthiophen-3-yl)propyl p-toluenesulfonate 6.0mmol, 1-(3'-fluorobiphenyl-4-yl)piperazine 5.0mol, sodium carbonate 15.0 mol and 0.4 mol of potassium iodide were added to 80 mL of acetonitrile, stirred and reacte...
Embodiment 2
[0077] Example 2: Preparation of 5-cyclobutyl-2-(4-(3-(5-dichloromethyl-thiophen-3-yl)-3-methylpiperazin-1-yl)pyrimidine (compound 2)
[0078]
[0079] Step 1. Dissolve 10.0 mmol of 3-(5-dichloromethyl-thiophen-3-yl)propan-1-ol in 100 mL of chloroform, and add 3 mL of triethylamine and 12.0 mmol of p-toluenesulfonyl chloride to it in sequence. After stirring the reaction at room temperature for 4 hours, 200 mL of water was added to quench. The organic phase was separated, dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure, and the resulting residue was purified by silica gel column chromatography (cyclohexane / ethyl acetate (v / v)=4 / 1) to obtain 3-(5 -Dichloromethyl-thiophen-3-yl)propyl p-toluenesulfonate 3.19 g (84.3%).
[0080] Under nitrogen protection, 3-(5-dichloromethyl-thiophen-3-yl)propyl p-toluenesulfonate 6.0mmol, 5-cyclobutyl-2-(3-methyl-pipera-1- Base) 5.0 mol of pyrimidine, 15.0 mol of sodium carbonate and 0.4 mol of potassiu...
Embodiment 13
[0086] Embodiment 13: detection of aldose reductase activity
[0087] Refer to the literature method (Bovine lens aldehyde reductase (aldose reductase). Purification, kinetics and mechanism, A B, Halder; M J, Crabbe, The Biochemical journal, 1984, volume 219, phase 1, 33-39)
[0088] Take the rat lens and place it in homogenization buffer (135mM KH 2 PO 4 -NaH 2 PO 4 , 120mM Li 2 SO 4 , pH7.0), homogenate at 10000g, centrifuge at 40C for 20min, absorb the supernatant, and obtain the enzyme crude extract. Enzyme activity and sample activity detection methods are as follows, add buffer (0.1M KH 2 PO 4 , pH7.0), an appropriate amount of enzyme and the sample to be tested, mix well, measure the background OD value of the drug at 340nm (recorded as OD0 value), then add 0.15mM NADPH and 5mMDL-glyceraldehyde at 37°C for 10min , 20min, 340nm measured OD value (denoted as OD1 value), calculated inhibition rate and IC 50 . When measuring the activity, the sample with a concent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com