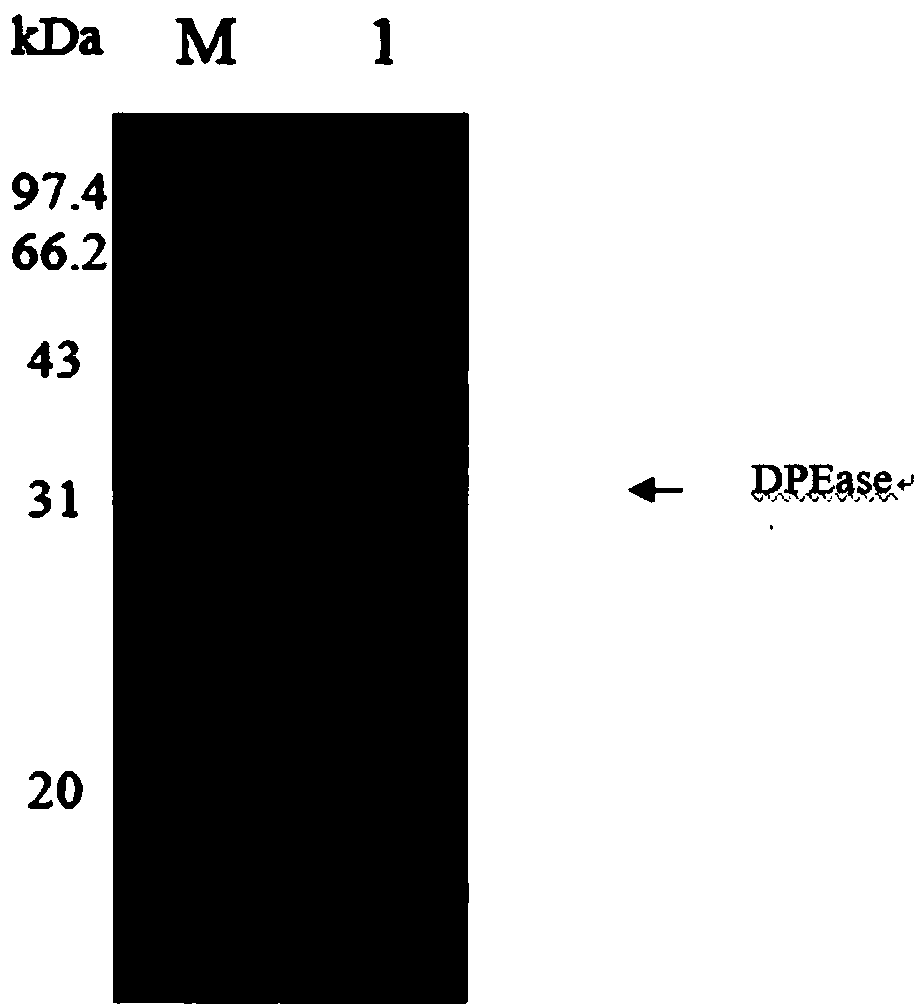
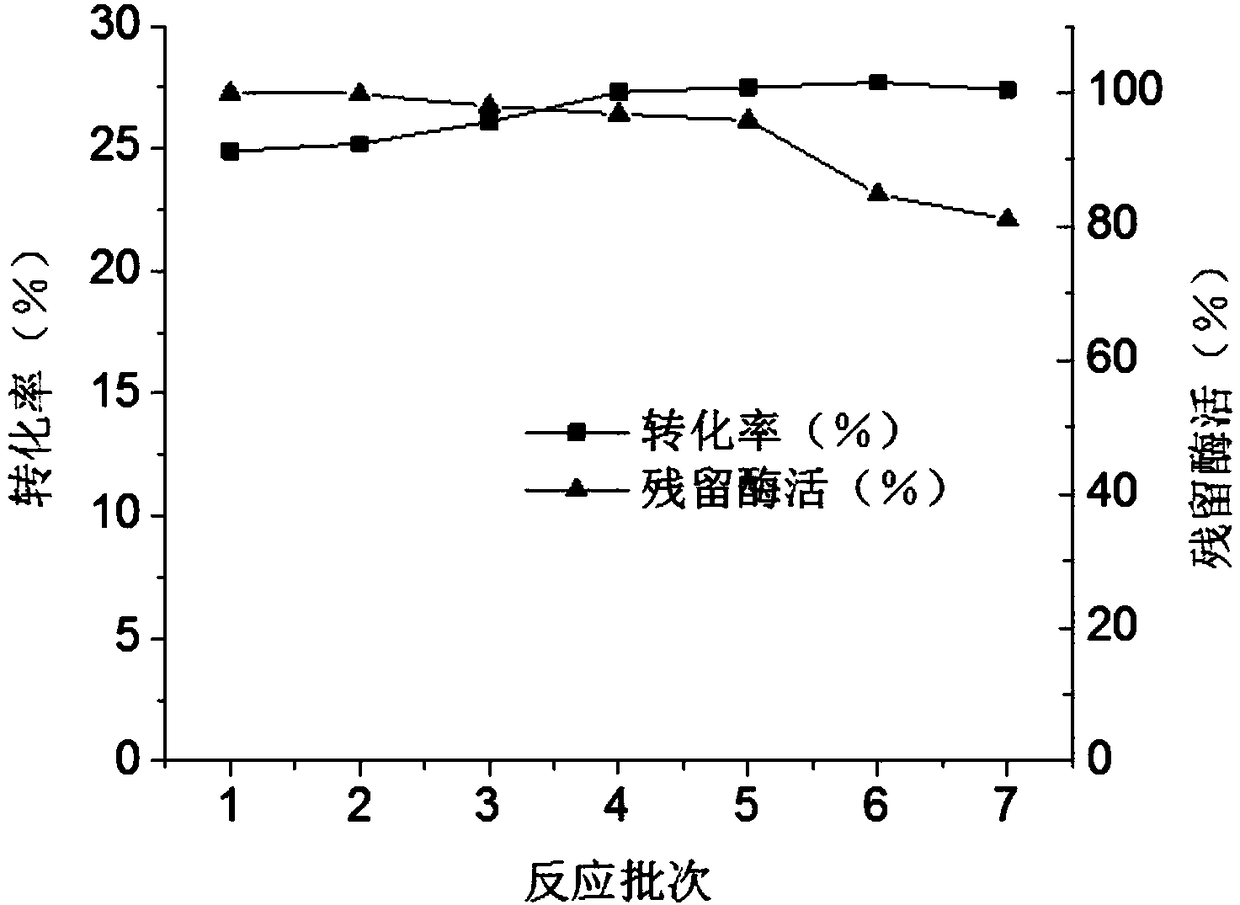
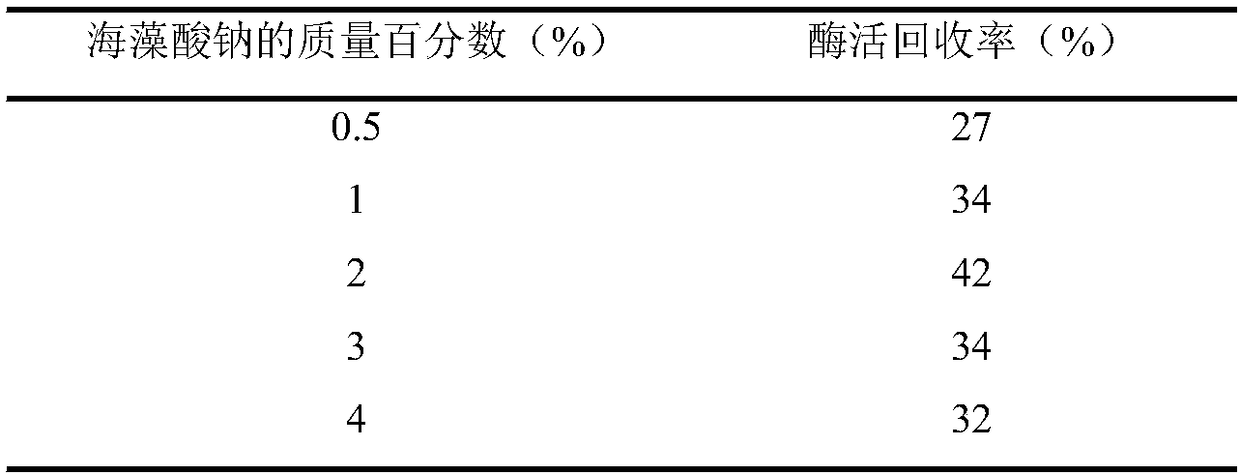
D-psicose 3-epimerase production strain and immobilization method thereof
A technology of epimerase and psicose, which is applied to the production strain of D-psicose 3-epimerase and the field of immobilization thereof, can solve the problem of high cost, unreusable enzyme liquid cost, Free enzymes cannot be reused, etc., to achieve the effect of simplified separation and purification, good continuous transformation ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: Cloning of Clostridium cellulolyticum DPEase coding gene and construction of expression vector
[0031] Two pairs of primers P1 and P2, P3 and P4 with 15bp homology arms were designed according to the DPEase gene in the database and the Escherichia coli-Bacillus subtilis shuttle vector pHY300PLK sequence:
[0032] P1: 5'-TAAGGAGTGTCAAGAATGAAACATGGCATC-3'
[0033] P2: 5'-TTTATTACCAAGCTTTTAGCTATGTTTATGA-3'
[0034] P3: 5'-TCATAAACATAGCTAAAAGCTTGGTAATAAA-3'
[0035] P4: 5'-GATGCCATGT TTCATTCTTGACACTCCTTA-3'
[0036] Using the plasmid with the DPEase gene and the pHY300PLK vector as templates, clone the target gene containing 15bp homology arms and the pHY300PLK vector, recover the PCR products separately, and use infusion for seamless connection. Connection system: target gene 2μL, pHY300PLK 0.5μL, infusion 1μL, ddH 2 O 1.5 μL. After mixing the above system, place it in a water bath at 50°C for incubation for 30 minutes, then immediately transfer it to a...
Embodiment 2
[0037] Embodiment 2: Transformation of recombinant plasmid pHY300PLK-DPEase
[0038] 1) Pick a single colony of Bacillus subtilis (CCTCC M2016536) on a fresh LB plate (LB solid medium g / L: peptone 10, yeast extract 5, NaCl 10, 0.2 agar powder) and inoculate it in 10 mL LB liquid medium, 37 Cultivate at 200rpm for 10.5h.
[0039] 2) Take 2.5 mL of the overnight culture and transfer it into 40 mL of LB medium added with 0.5 M sorbitol, culture at 37° C. with shaking at 200 rpm for 4.5 h.
[0040] 3) Cool the bacterial solution on ice for 10 minutes, then centrifuge at 5000 rpm at 4°C for 5 minutes to collect the bacterial cells.
[0041] 4) Wash the cells with 50 mL pre-cooled electroporation medium (sorbitol 0.5 M, mannitol 0.5 M, glucose 10%), centrifuge at 5000 rpm, 4° C. for 5 min to remove the supernatant, and rinse 4 times in this way.
[0042] 5) Resuspend the washed bacteria in 1 mL of electroporation medium, aliquot them into 1.5 mL EP tubes, and fill each tube with 2...
Embodiment 3
[0045] Embodiment 3: Shake flask fermentation produces enzyme
[0046] 1) Fermentation culture
[0047] The recombinant Bacillus subtilis BS-pHY300PLK-DPEase strain obtained in Example 2 was inoculated in LB medium, and after cultivating at 37°C for 8-10h, it was transferred to TB fermentation medium with 5% inoculum size, at 33°C, Incubate at 200 rpm for 48 hours to produce enzyme. After the fermentation, the wall was ultrasonically broken to obtain the crude enzyme liquid.
[0048] LB medium (g / L): peptone 10, yeast extract 5, NaCl 10
[0049] TB medium (g / L): peptone 10, yeast powder 24, glycerol 5, K 2 HPO 4 ·3H 2 O 16.43, KH 2 PO 4 2.31.
[0050] 2) Determination of enzyme activity
[0051] Add 200 μL of appropriately diluted enzyme solution to 800 μL of fructose-containing HEPES buffer (20 mM / L, pH 8.0, 0.1 mmol / L Co 2+ ), make the final concentration of fructose 80g / L. After shaking and mixing, place in a 55°C water bath to react for 10 minutes, and boil for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com