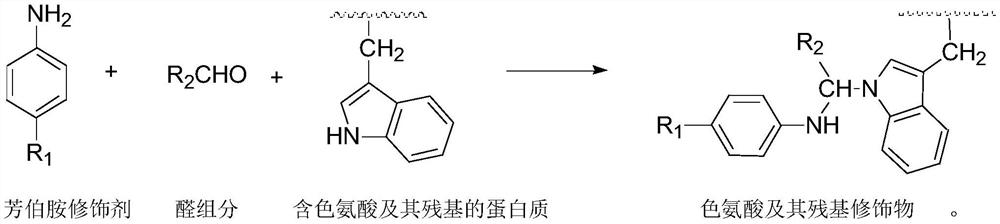
A highly selective chemical modification method targeting tryptophan and its residues
A high-selectivity, chemical modification technology, applied in chemical instruments and methods, peptide preparation methods, organic chemistry, etc., can solve the problems of poor selectivity, difficult to achieve chemical modification of amino acid residue sites, etc., to achieve small impact , excellent durability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
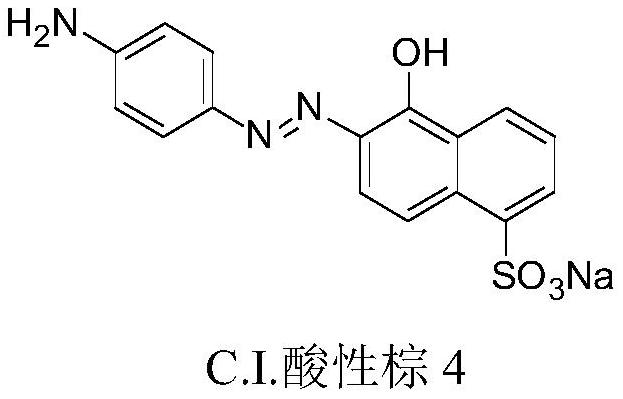
[0024] Embodiment 1, a highly selective chemical modification method for tryptophan and its residues, select the primary arylamine dye C.I. Acid Brown 4 as the primary arylamine modifier, formaldehyde as the aldehyde component, tryptophan, tyrosine, Ten highly active amino acids including histidine, cysteine, serine, threonine, arginine, aspartic acid, lysine, and phenylalanine were used as modified substances.
[0025]
[0026] The chemical modification method is as follows:
[0027] Select 37% (mass %) formaldehyde solution for use.
[0028] Mix C.I. acid brown 4, amino acid, and formaldehyde (aqueous solution) in a molar ratio of 1:1:100. At this time, the mass ratio of water to primary aramide modifier is about 50:1; the pH value of the reaction solution is adjusted to 4.0 (use 3.6 mL of 1mol / L sodium acetate and 16.4mL of 1mol / L acetic acid are prepared, which is a conventional technique), the reaction temperature is 30°C, and the holding time is 24 hours.
[0029] A...
Embodiment 2
[0034] Embodiment 2, a highly selective chemical modification method for tryptophan and its residues, using p-methylaniline as the primary aromatic amine modifier, acetaldehyde as the aldehyde component, and polypeptide A containing tryptophan residues ( The amino acid sequence is Tyr-Glu-Tyr-Ala-Trp-Ser-Ser, wherein Trp is tryptophan, Tyr is tyrosine, Glu is glutamic acid, Ala is alanine, Ser is serine) as the modified substance .
[0035] The chemical modification method is as follows:
[0036] 0.007mmol of polypeptide A, 0.05mmol (5.35mg) of p-methylaniline and 0.15mmol of acetaldehyde; the water in the reaction system was 35mL; the pH value was 4.5, the reaction temperature was 37°C, and the holding time was 22 hours.
[0037] After the reaction, wash with hot water and cold water (washed with deionized water at 60°C and 15°C for 10 h) to remove impurities adsorbed on the polypeptide, filter, wash (wash with water until the eluent is neutral), to dry. The analysis resul...
Embodiment 3
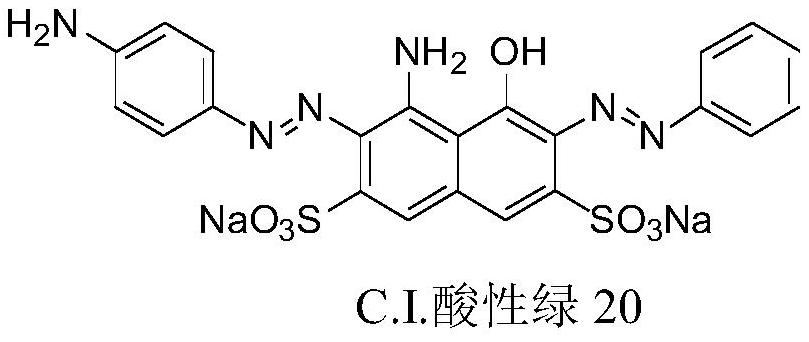
[0039] Example 3, a highly selective chemical modification method for tryptophan and its residues, using C.I. Acid Green 20 as the primary aryl amine modifier, the aldehyde component is methylglyoxal, and mulberry silk containing tryptophan residues as Modified object.
[0040]
[0041] The chemical modification method is as follows:
[0042] 0.35g mulberry silk fabric (containing about 0.007mmol tryptophan) is mixed with 7.5mg (0.07mmol) of C.I. Acid Green 20 and 7.0mmol of methylglyoxal; the water in the reaction system is 15mL; the pH value is 5.0, and the reaction temperature is 30 ℃, holding time 20 hours.
[0043] After the reaction, wash with deionized water at 60° C. and 15° C. for 10 hours each to remove impurities adsorbed on the silk, and dry in the air.
[0044] After the reaction, the silk is dyed brown, and the fiber strength loss rate is measured to be less than 5%. DMF stripping method (100°C, 20min) proved that the dyes covalently bonded with silk tryptoph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com