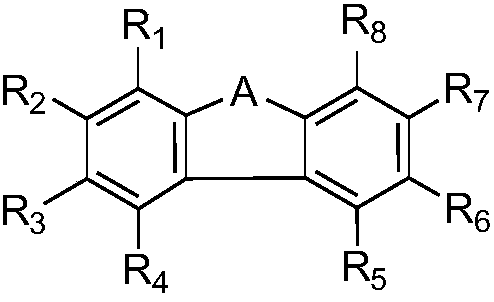
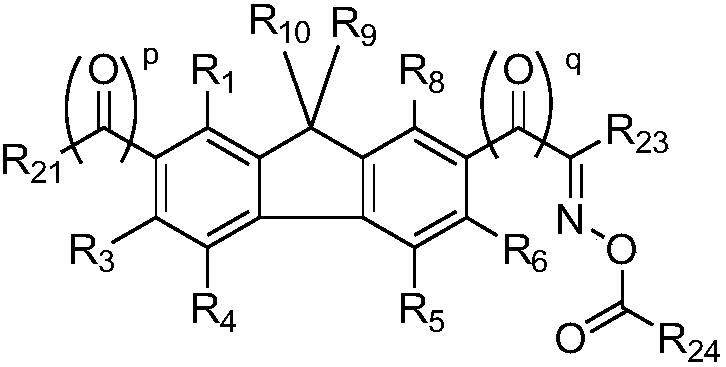
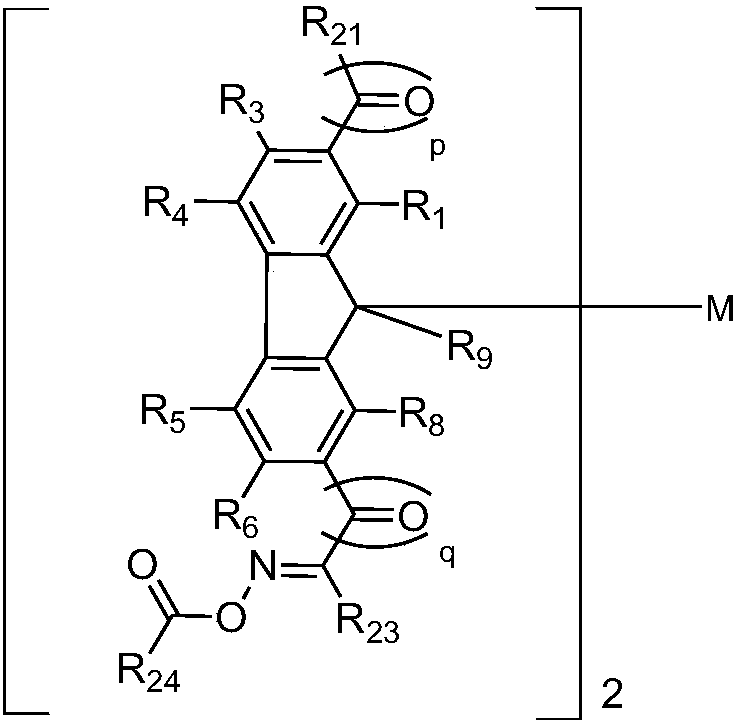
Fluorene derivative, and photopolymerization initiator and photoresist composition containing same
A derivative, C3-C30 technology, applied in the fields of photopolymerization initiators and photoresist compositions containing the same, fluorene derivatives, and new fluorene derivatives, which can solve the problem of low sensitivity and low photopolymerization initiator usage or Exposure, mask contamination, yield reduction, etc., to achieve the effects of excellent reactivity, improved film retention rate, and high solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] (Example 1) Preparation of 1-(9,9-diallyl-9H-fluoren-2-yl)-1,2-propanedione-2-oxime-O-acetate (1)
[0109] Step 1. Preparation of 9,9-diallyl-9H-fluorene
[0110]Under a nitrogen atmosphere, dissolve 20.0g (120.32mmol) of fluorene, 27.0g (481.29mmol) of potassium hydroxide and 1.99g (12.03mmol) of potassium iodide in 140ml of anhydrous tetrahydrofuran, and after maintaining at 15°C, dissolve allyl bromide 30.57 g (252.67mmol) was diluted in 40ml of anhydrous tetrahydrofuran and added dropwise slowly. After the dropwise addition, the mixture was stirred at normal temperature (23° C.) for 1 hour. After slowly pouring into 300 ml of ice-distilled water to terminate the reaction, the product was extracted with 500 ml of dichloromethane. The organic layer was separated and washed with water twice. After drying the organic layer with anhydrous magnesium sulfate, the anhydrous magnesium sulfate was separated by filtration. The product obtained by concentrating the filtrate...
Embodiment 2
[0121] (Example 2) 1-(9-(3-methylenecyclohexyl)-9H-fluoren-2-yl)-1,2-propanedione-2-oxime-O-acetate (4)
[0122] Step 1. Preparation of 9-(3-cyclohexanonyl)-9H-fluorene
[0123] Under a nitrogen atmosphere, 10.0 g (60.16 mmol) of fluorene and 14.96 g (61.36 mmol) of 1,5-dibromopentan-3-one were dissolved in 350 ml of anhydrous tetrahydrofuran, and after maintaining at 5° C., 0.99 g of potassium iodide ( 6.01 mmol). 13.50 g (240.64 mmol) of potassium hydroxide was added bit by bit while preventing heat generation. After charging, the reactant was heated up to 15° C. and stirred for 1 hour, and then stirred at room temperature for 1 hour. After slowly pouring into 500 ml of ice-distilled water to terminate the reaction, the product was extracted with 300 ml of dichloromethane. The organic layer was separated and washed with water twice. After drying the organic layer with anhydrous magnesium sulfate, the anhydrous magnesium sulfate was separated by filtration. The product o...
Embodiment 3
[0131] (Example 3) 1-(9-(2-cyclopentenyl)-9H-fluoren-2-yl)-1,2-propanedione-2-oxime-O-acetate (6)
[0132] In step 1 of the above example 2, 1,4-dibromo-2-butene was used instead of 1,5-dibromopentan-3-one, except that, the same method was used to carry out the Step 1, prepare 9-(2-cyclopentenyl)-9H-fluorene, then use 9-(2-cyclopentenyl)-9H-fluorene instead of 9,9-di Allyl-9H-fluorene, except that, using the same method to carry out steps 2 to 4 in Example 1, to obtain light yellow solid 1-(9-(2-cyclopentenyl)-9H-fluorene -2-yl)-1,2-propanedione-2-oxime-O-acetate 3.18 g (66%).
[0133] 1 H-NMR (δppm: CDCl 3 ):2.20(3H,s),2.34(3H,s),2.62-2.76(4H,d),5.71-5.82(2H,d),7.35-7.48(3H,m),7.76-7.88(2H,d ),8.11-8.22(2H,d)
PUM
| Property | Measurement | Unit |
|---|---|---|
| dispersity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com