Pyrone compounds and preparation method and application thereof
A compound and pyrone technology, which is applied in the fields of genetic engineering and biopharmaceuticals, can solve the problems such as no antiviral activity of violapyrone compounds, and achieve the effects of low toxicity, huge market space and good activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Cloning of polyketide synthase gene
[0044] 1. Genomic DNA extraction
[0045] Inoculate marine Streptomyces ZH66 in TSBY liquid medium, culture overnight at 30°C, collect the cells by centrifugation, wash with an appropriate amount of STE buffer; add 3-5 mg / ml lysozyme solution prepared by STE buffer, carefully suspend the cells, In 37°C water bath for 30min, until the cells become translucent; add 6% SDS, mix gently up and down, continue to 37°C water bath until clear; add appropriate amount of 3M NaAc (pH=4.8), then add appropriate amount of phenol:chloroform : isoamyl alcohol (25:24:1; v / v / v), mixed, and centrifuged at 12000rpm; pipette the supernatant, and repeatedly extract with phenol: chloroform: isoamyl alcohol until the middle layer is free of protein impurities, transfer to Add an equal volume of isopropanol and mix until a white flocculent DNA precipitates out; pick out the flocculent precipitate and wash it with 70% ethanol for 1-2 times; after...
Embodiment 2
[0054] Embodiment 2: Preparation of α-pyrone compound 1-4
[0055] 1. Fermentation production
[0056] (1) Cultivation of spores: According to the conventional method for cultivating microorganisms, an appropriate amount of recombinant strain I was inoculated onto MS solid slant medium and placed in a constant temperature incubator at 30° C., and cultivated for 3-4 days.
[0057] MS medium: 20g soybean powder, 20g mannitol, 20g agar powder, dissolved in water, constant volume to 1L, sterilized at 121°C for 30 minutes. After sterilization, pour the culture medium into a petri dish with a diameter of 90mm, and distribute it according to 30ml / plate.
[0058] (2) Fermentation culture
[0059] Take an appropriate amount of spores of the recombinant strain cultured on a slant for 3-4 days, inoculate them into a 250ml Erlenmeyer flask filled with 50ml of culture medium, place them on a constant temperature shaker at 30°C, and cultivate them at a speed of 220rpm for 7 days to obtain...
Embodiment 3
[0064] Example 3: Characterization of Compounds 1-4
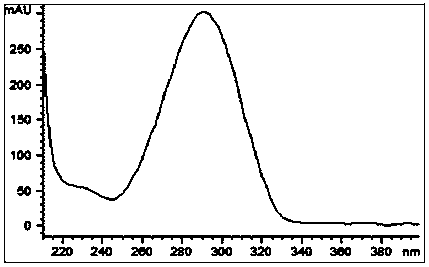
[0065] Compound 2 Light yellow amorphous solid, UV(MeOH)(logε)λ max 290.0(3.72), molecular formula C 14 h 22 o 3 , HR-ESIMS m / z 239.1640[M+H] + . in, 1 H and 13 See Table 1 for C-NMR data.
[0066] Table 1 Compound 2 1 H and 13 C NMR data (500 and 150 MHz, in d 6 -DMSO) a
[0067]
[0068]
[0069] The signal assignments in Table 1 are based on the analysis results of H-H COZY, HMQC and HMBC spectra. The multiplicity of carbon signals is represented by s (singlet), d (doublet), t (triplet), q (quartet) and m (multiplet), respectively.
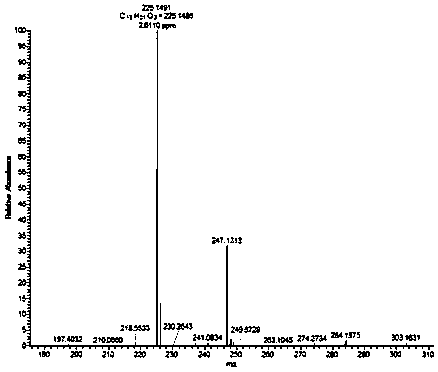
[0070] Compound 1 is a light yellow amorphous solid, molecular formula C 13 h 20 o 3 , HR-ESIMS m / z 225.1483[M+H] + .
[0071] Compound 3 is light yellow amorphous solid, molecular formula C 14 h 22 o 3 , HR-ESIMS m / z 239.1640[M+H] + .
[0072] Compound 4 is light yellow amorphous solid, molecular formula C 14 h 22 o 3 , HR-ESIMS m / z 239.1638[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com