2,3-disubstituted boron-containing indole compounds with high enantioselectivity and preparation method of 2,3-disubstituted boron-containing indole compounds
An enantioselective, disubstituted technology, applied in the field of high enantioselective 2,3-disubstituted boron-containing indole compounds and their preparation, can solve the problems that have not been reported in the literature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The present invention also provides a method for preparing highly enantioselective 2,3-disubstituted boron-containing indole compounds, the method comprising:
[0043] Under the protection of nitrogen, add catalyst, ligand (S, S)-Ph-BPE, alkali and solvent into the reaction vessel, and stir at room temperature. The stirring time is preferably 8-15 min, more preferably 10 min, and then add the coupling The pinacol borate is stirred continuously, the stirring time is preferably 8-15 min, more preferably 10 min, and then functionalized o-iminostyrene is added, preferably after stirring for 5 min, and then methanol is added to react at room temperature. The reaction time is 6-8h, and the completion of the reaction is detected by TLC. Then, after extraction, the organic phases are combined, dried with anhydrous sodium sulfate, filtered with suction, and distilled under reduced pressure to remove the organic solvent, and finally subjected to silica gel column chromatography to ob...
Embodiment 1
[0051]
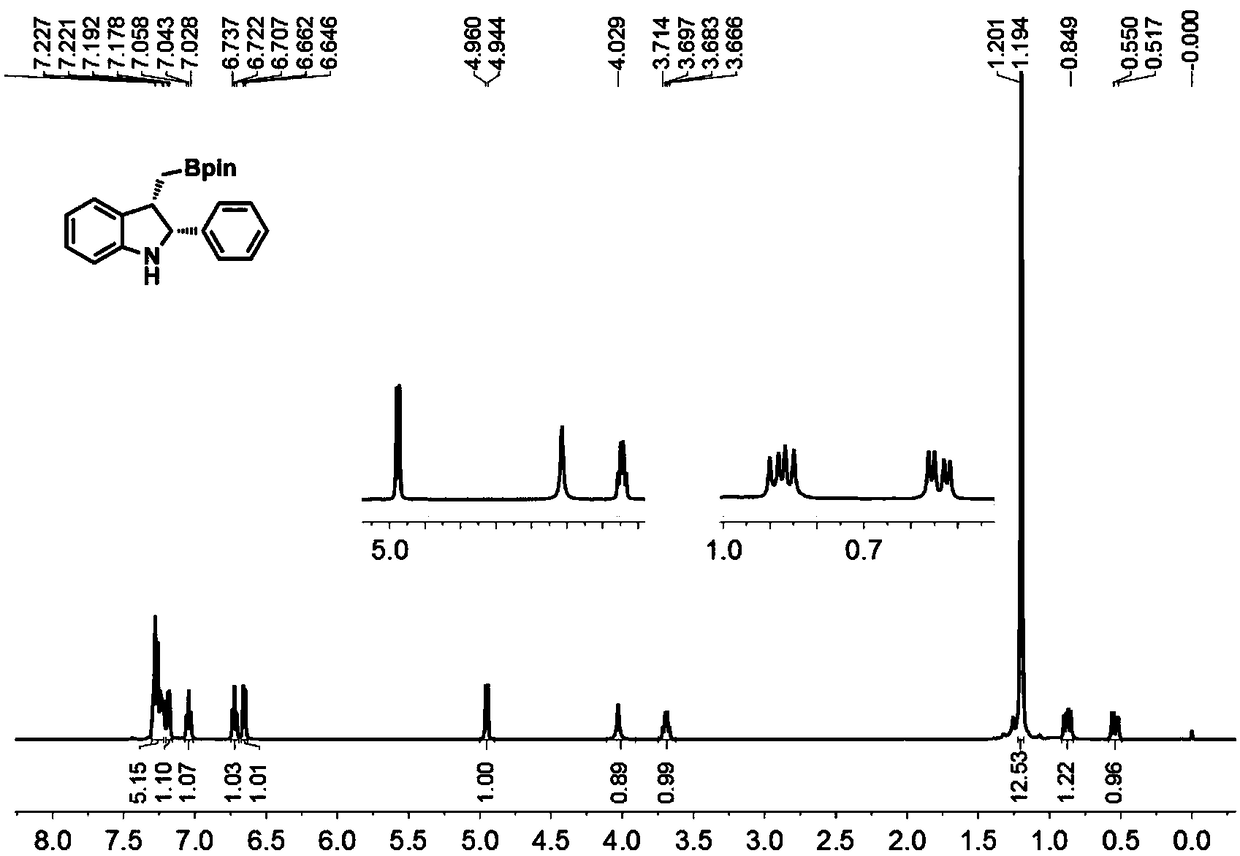
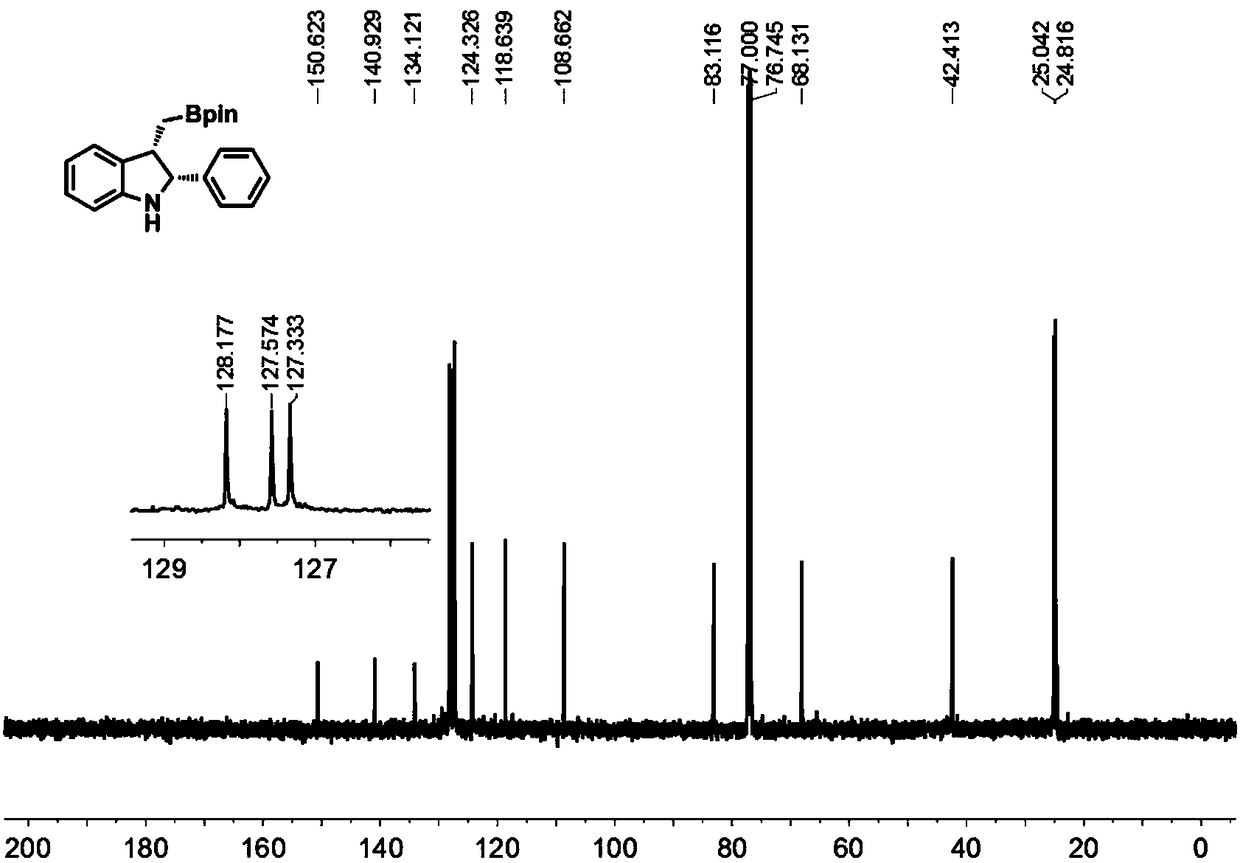
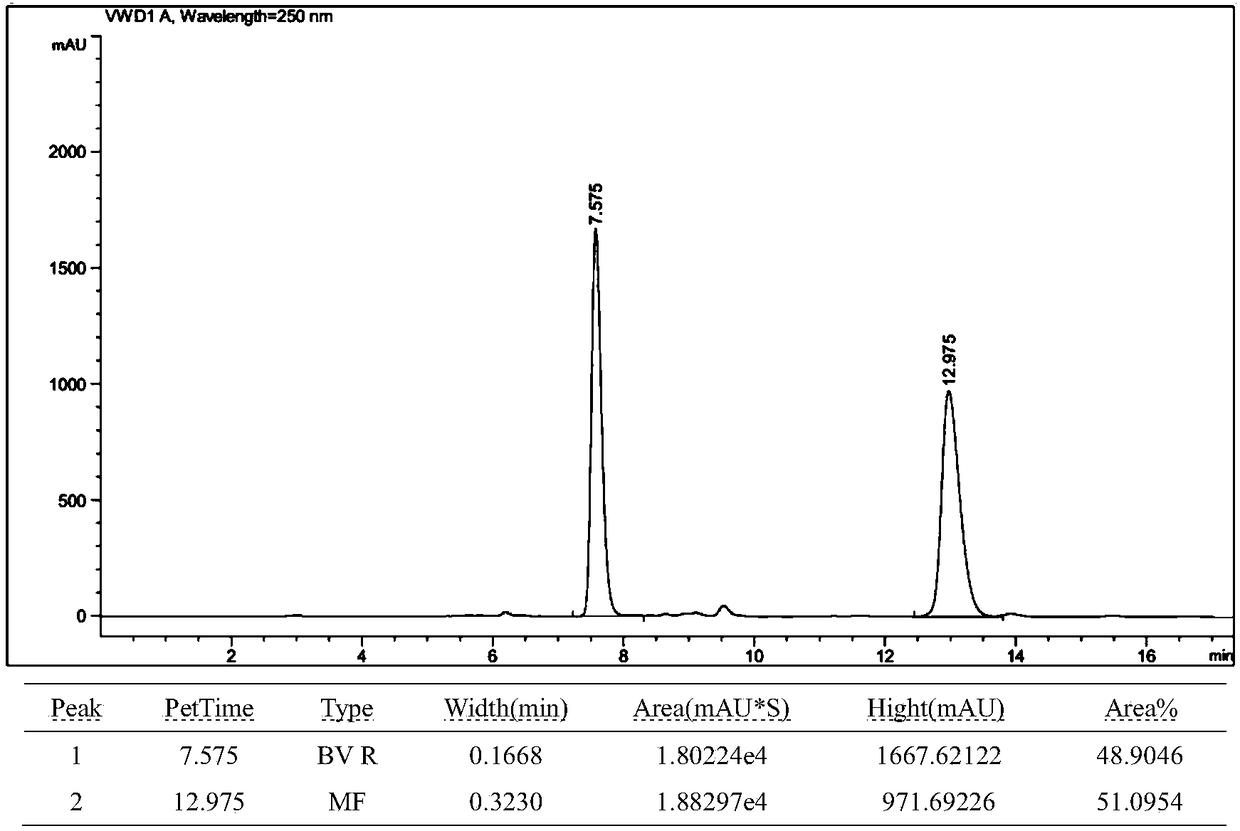
[0052] Under the protection of nitrogen, add cuprous chloride (2mol%), (S,S)-Ph-BPE (3mol%) and NaOtBu (0.30mmol) to a 10mL pressure-resistant sealed tube with a stir bar, and then add tetrahydrofuran ( 2mL), stirring at room temperature for 10 min. After stirring for 10 min, add pinacol diboronic acid (0.30 mmol) to the system, continue to stir for 10 min, then add 1a (0.20 mmol), stir for 5 min, then add methanol (0.20 mmol), leave the reaction at room temperature, stir for 8 h, and use TLC detects the completion of the reaction. The reaction was quenched with water (10ml), extracted with dichloromethane (3×10mL), the organic phases were combined, dried with anhydrous sodium sulfate, filtered with suction, and distilled under reduced pressure to remove the organic solvent, and finally subjected to silica gel column chromatography to obtain 2,3 -Disubstituted indole compound 2a, with a yield of 96%.
[0053] figure 1 For the 2,3-disubstituted indole 2a prepared in Exa...
Embodiment 2
[0057] Replace 1a in the example with 1b, and other conditions are the same as in Example 1, to obtain 2,3-disubstituted indole compound 2b with an ee value of 98%. .
[0058]
[0059] The hydrogen nuclear magnetic spectrum data, the carbon nuclear magnetic spectrum data and the high performance liquid chromatography data of the product 2b prepared in Example 2 are as follows:
[0060] White solid.NMR Spectroscopy: 1 H NMR(500MHz, CDCl 3 )δ=7.17(d,J=8.0Hz,3H), 7.07(d,J=8.0Hz,2H), 7.04(d,J=7.5Hz,1H), 6.72(t,J=7.5Hz,1H) ,6.66(d,J=7.5Hz,1H), 4.93(d,J=8.5Hz,1H),4.01(s,1H),3.66(q,J 12 =8.5Hz, J 13 = 15.5Hz, 1H), 2.31 (s, 3H), 1.20 (d, J = 5.0 Hz, 12H), 0.90-0.85 (m, 1H), 0.58-0.54 (m, 1H); 13 C NMR(125MHz, CDCl 3 )δ=150.66,137.82,136.88,134.19,128.82,127.46,127.26,124.28,118.53,108.60,83.60,67.87,42.35,25.02,24.80,21.07.Mass Spectrometry:HRMS(ESI-TOF)(m / z): Calcd for C 22 H 29 BNO 2 ([M+H] + ),350.2290,found,350.2290.[α] D 19 =+15.8,(c=1,CHCl 3 ).HPLC analysis(AD-H,5%IPA / hexane,1mL / min...
PUM
| Property | Measurement | Unit |
|---|---|---|
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com