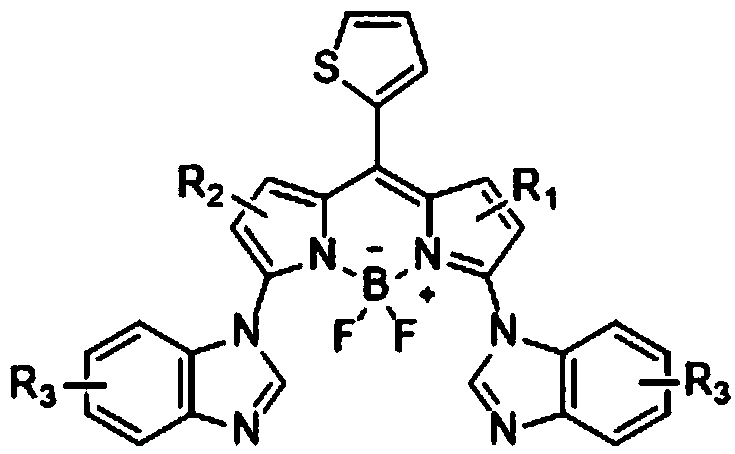
3, 5-dibenzimidazolyl-8-thiophenyl fluoroboron fluorescent derivatives as well as preparation method and application thereof
A thienyl fluoroboron and benzimidazole-based technology, which is applied in the field of fluoroborin derivatives, can solve the problems of complex structure, inconvenient synthesis, and high price of endoplasmic reticulum targeting reagents, and achieves easy mass acquisition and low cost , cheap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Synthesis of 3,5-Dibenzimidazolyl-8-thienylfluoroborofluorene
[0051] Add 8-thienylfluoroborate (13.7mg, 0.05mmol), benzimidazole (23.6mg, 0.20mmol), AgOAc (33.4mg, 4.0equiv), dimethyl sulfoxide (1.0mL) into the reaction tube, and Stir evenly under nitrogen, heat to 80°C, and react for 12 hours; the specific reaction formula is as follows:
[0052]
[0053] After the reaction is complete, cool the reaction tube to room temperature, remove the solvent and add 10 mL of dichloromethane to dissolve the reaction system, then filter through diatomaceous earth and wash with 10-20 mL of dichloromethane, combine the filtrates, remove the solvent under reduced pressure, The residue was separated and purified by silica gel column chromatography (dichloromethane / petroleum ether / ethyl acetate=10:10:1, v / v / v), and after vacuum drying, the target product 3,5-dibenzimidazole was obtained as a black solid 5.1 mg of yl-8-thienylfluoroborin, yield 20%.
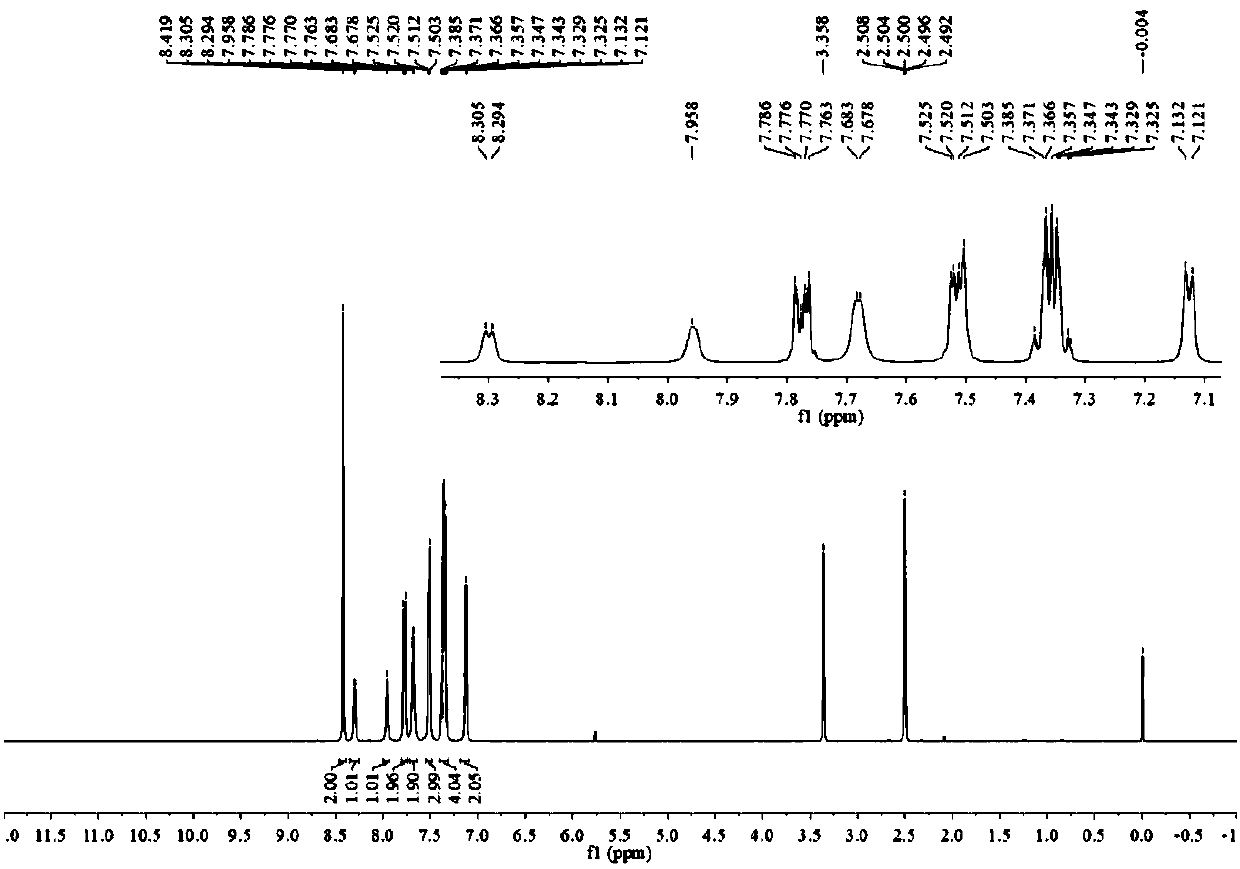
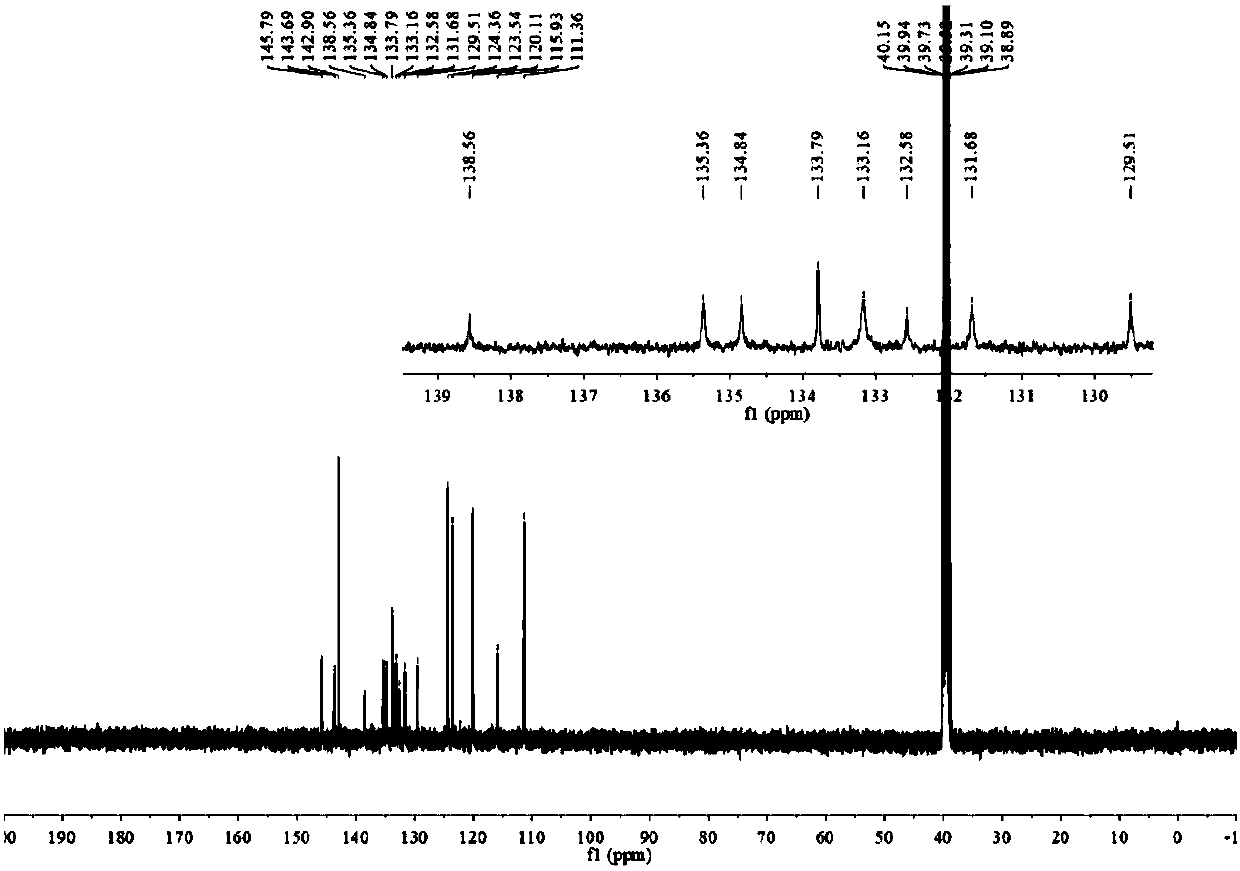
[0054] The H spectrum and C ...
Embodiment 2
[0057] The ultraviolet-visible-near-infrared absorption spectrum and the fluorescence emission spectrum of the compound 3,5-dibenzimidazolyl-8-thienylfluoroborate prepared in Example 1
[0058] The compound 3,5-dibenzimidazolyl-8-thienylfluoroboron was dissolved in dichloromethane to form 1×10 -5 mol / L, take 2.5mL and put it into a cuvette, and measure the UV-visible-near-infrared absorption and fluorescence emission spectra.
[0059] Figure 4 It is the UV-visible absorption spectrum and fluorescence emission spectrum of the compound 3,5-dibenzimidazolyl-8-thienylfluoroborate, wherein the black solid line represents the UV-vis absorption spectrum, and the black dotted line represents the fluorescence emission spectrum. from Figure 4 It can be seen from the figure that the maximum absorption peak of the compound 3,5-dibenzimidazolyl-8-thienylfluoroborate is located at 559nm; the maximum absorption peak of the fluorescence emission spectrum is located at 590nm, and the Stoke...
Embodiment 3
[0061] The compound 3,5-dibenzimidazolyl-8-thienylfluoroborate prepared in Example 1 and the commercially available endoplasmic reticulum stain ER-Tracker TM Fluorescence confocal confocal imaging of Green in HepG2 cells
[0062] First, add 5% CO to the DMEM(H) medium containing 10% fetal bovine serum 2 , HepG2 cells were cultured at 37°C for 24 hours. The medium was removed, and 2.5 μM of the compound 3,5-dibenzimidazolyl-8-thienylflubororin in phosphate buffer was added, followed by 1 μM of the commercially available endoplasmic reticulum stain ER-Tracker TM Green was co-incubated at 37°C for 30 minutes. After the culture is over, take out the culture glass-bottom dish, wash it with phosphate buffer saline for 2 to 3 times, and image the culture glass-bottom dish through a fluorescent confocal microscope.
[0063] Figure 5 It is the fluorescence imaging image of the compound 3,5-dibenzimidazolyl-8-thienylfluoroborate (excitation wavelength: 553nm, emission wavelength ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com