Method for catalyzing lignin to select depolymerization by using non-precious metals
A non-precious metal and lignin technology, applied in the field of Mn complex catalysts, can solve the problems of consuming hydrogen or oxygen sources, harsh reaction conditions, and polluting the environment, and achieve the effects of low cost, mild reaction conditions, and pollution reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
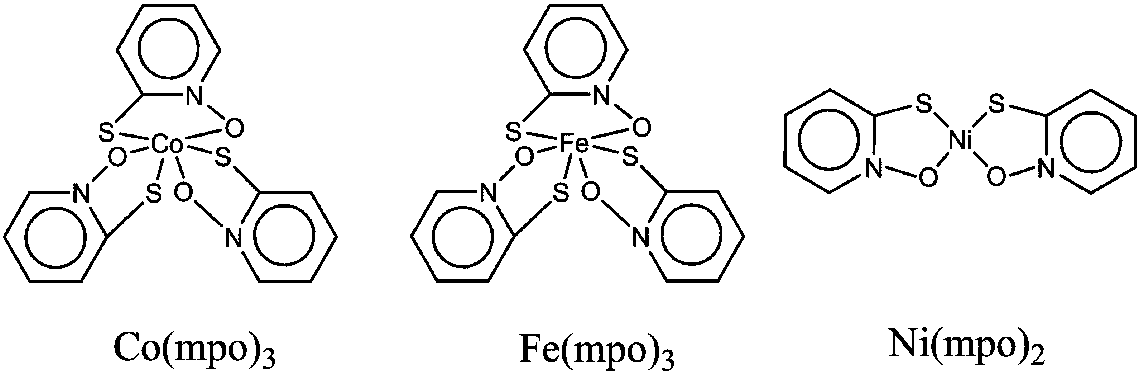
[0024] M(mpo) n The specific preparation method of the complex catalyst (M=Fe, Co, n=3; M=Ni, n=2) is as follows:
[0025] Fe(mpo) 3 Preparation: 1.0g anhydrous ferrous chloride, 3.0g 2,2'-dithiobis(pyridine-1-oxide), 0.43g sodium methoxide in 200ml methanol, stirred at room temperature for 30h, filtered after the reaction, blue The brown filtrate was refrigerated overnight at 5°C to obtain crystals, the liquid was separated, the collected crystals were dried in vacuum to obtain 2.41g of brown solid catalyst Fe(mpo) 3 , Yield: 70%.
[0026] Co(mpo) 3 Preparation: 2.0g of anhydrous cobalt chloride, 2.3g of 2-mercaptopyridine sodium salt were stirred in 300ml of tetrahydrofuran at room temperature for 24h, then filtered, and the brown-green filtrate was refrigerated at 5°C overnight to obtain crystals, the liquid was separated, and the crystals were collected Vacuum drying to get 5.28g solid catalyst Co(mpo) 3 , mol yield: 77%.
[0027] Ni(mpo) 2 Preparation: 0.38 g of 2,...
Embodiment 2-10
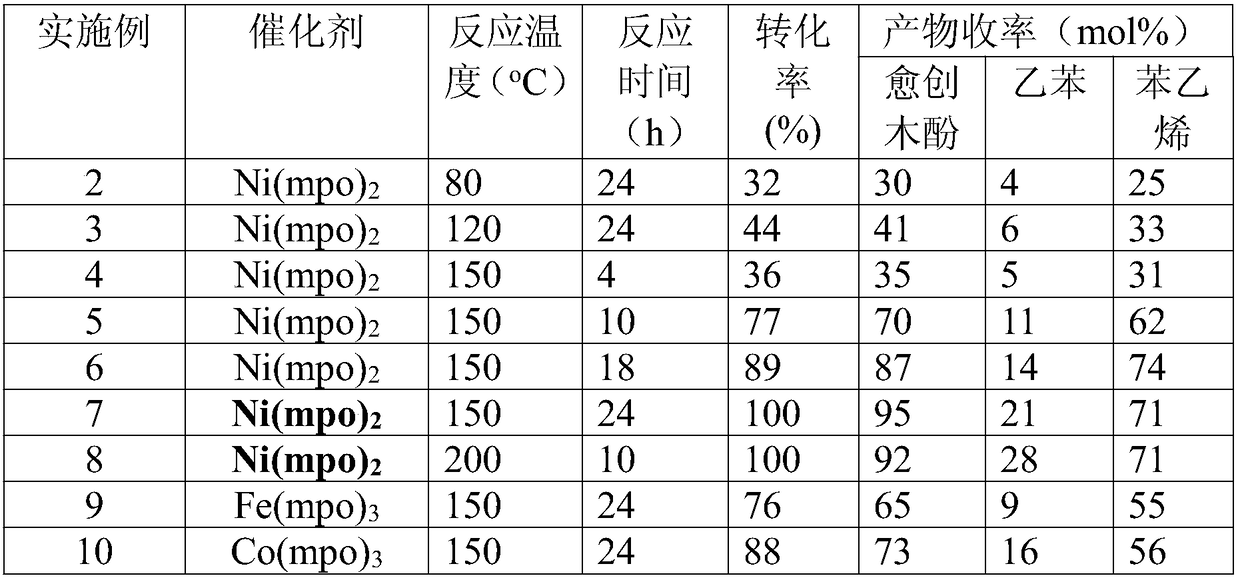
[0029] M(mpo) n The complex catalyzes the depolymerization reaction of the lignin model molecule 2-(2-methoxyphenoxy)-1-phenylethanol: mix 50mg lignin model molecule with M(mpo) n 5 mg of the complex was dissolved in 10 ml of methanol respectively, replaced with nitrogen five times, and then the reaction vessel was sealed under normal pressure and heated to 80°C-200°C, and the reaction was stirred at 1000 rpm for 4h-24h. After the reaction was completed, the temperature was lowered to room temperature, and the supernatant was filtered and then sampled for analysis. Qualitative analysis of the product was carried out by GC-MS technology and standard sample control, and quantitative analysis was realized by gas chromatography internal standard method. The reaction results are shown in Table 1.
[0030] Table 1 M(mpo) under different conditions n Depolymerization results of the complex catalyzed lignin model molecule 2-(2-methoxyphenoxy)-1-phenylethanol
[0031]
[0032] A...
Embodiment 11-16
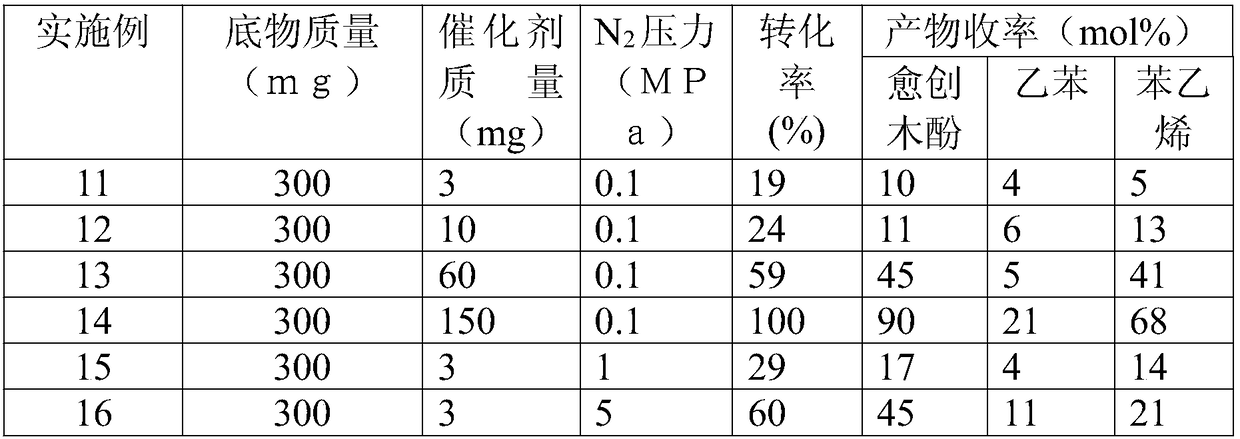
[0034] Ni(mpo) 2 Catalyze the depolymerization reaction of lignin model molecule 2-(2-methoxyphenoxy)-1-phenylethanol under different conditions: a certain mass of lignin model molecule and M(mpo) n The complexes were respectively dissolved in 30ml of methanol, replaced with nitrogen for five times, then filled with nitrogen to the set pressure, the temperature of the reactor was raised to 150°C, and the stirring reaction was carried out at a speed of 1000 rpm for 24 hours. After the reaction was completed, the temperature was lowered to room temperature, and the supernatant was filtered and then sampled for analysis. Qualitative analysis of the product was carried out by GC-MS technology and standard sample control, and quantitative analysis was realized by gas chromatography internal standard method. The reaction results are shown in Table 2.
[0035] Table 2 Ni(mpo) under different conditions 2 Depolymerization results of the complex catalyzed lignin model molecule 2-(2-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com