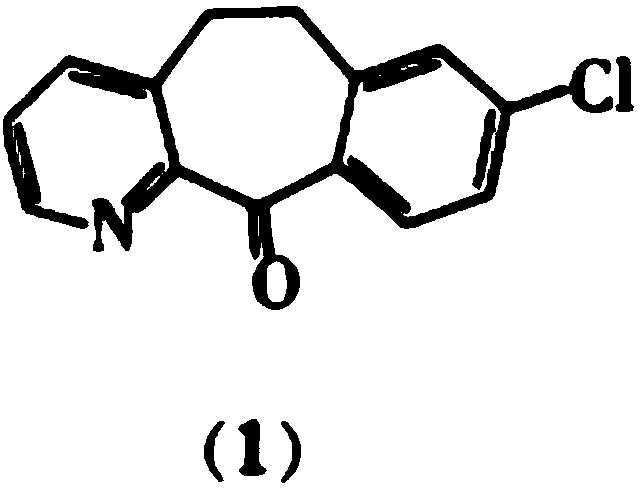
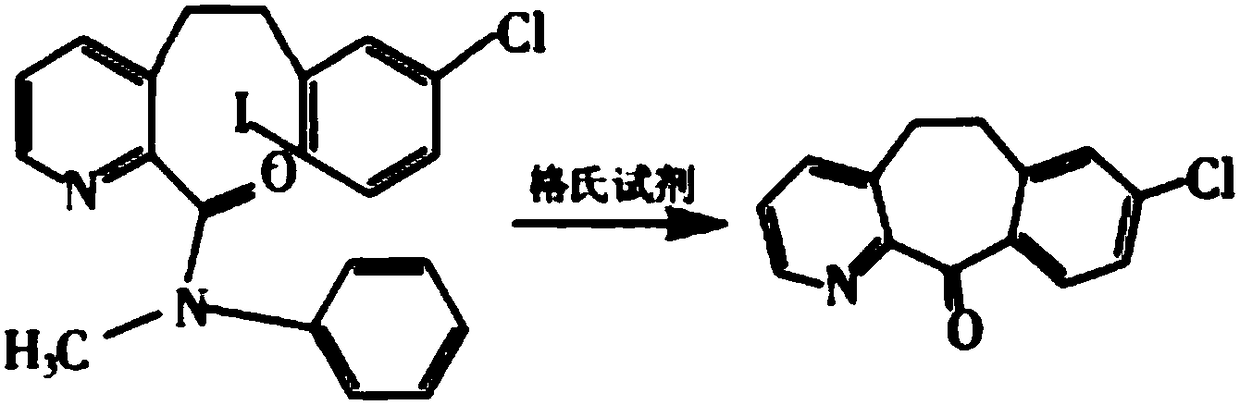
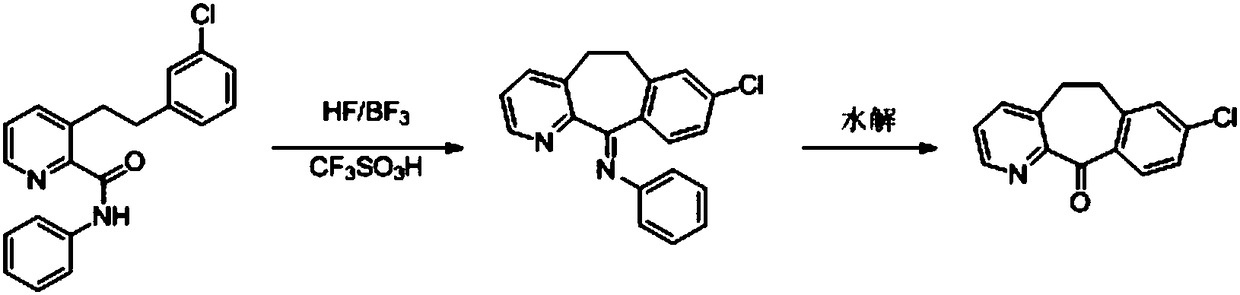
Method for preparing anti-allergic rhinitis medicine loratadine intermediate
A loratadine and anti-allergic technology, which is applied in the field of chemical preparation, can solve the problems of excessive addition, cumbersome post-processing, a large amount of waste acid, etc., and achieves the effect of reducing the pressure of environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Preparation of catalyst:
[0048] 1) Preparation of modified silica gel carrier: 10.0 g of high-purity silica gel (pore diameter of Particle size is 300-400 mesh) in 100ml of anhydrous toluene, heated to 40-60°C, then dropwise added 5ml of 3-(2-aminoethylamino)propyltrimethoxysilane and 10ml of 3-mercaptopropyl Trimethoxysilane, reflux reaction under nitrogen atmosphere for 8-10h; cool down to room temperature, filter, rinse with 20ml acetone, and dry at 80°C to obtain aminomercapto-modified silica gel; place aminomercapto-modified silica gel in 68%wt nitric acid aqueous solution Ultrasonic impregnation at 40-50°C for 1-2h, filter, wash with water until the filtrate becomes neutral, and vacuum-dry the filter cake at 65°C to constant weight to obtain sulfamic acid group-modified silica gel;
[0049] 2) Preparation of solid acid by co-precipitation: 14g ammonium metatungstate, 3.2g ZrOCl 2 "8H 2 Dissolve O in water and reflux for 1-2h, adjust the pH of the system to 8...
Embodiment 2
[0068] Adopt the catalyst prepared by embodiment 1 to investigate the impact of different reaction solvents, the preparation process is as follows:
[0069] a) Place 3.4g raw material amide SM and 0.5g catalyst in 20ml solvent, heat up to reflux reaction;
[0070] b) When HPLC detects that the raw material amide SM no longer decreases, cool down to room temperature and filter to remove the catalyst to obtain a filtrate;
[0071] c) adding 10ml of 2mol / L aqueous sodium hydroxide solution to the filtrate to adjust the pH to 10-11;
[0072] d) Heat up to 80-90°C and stir for 2-3 hours for hydrolysis, add dropwise 2mol / L hydrochloric acid aqueous solution to adjust the pH of the system to 6-7, add dropwise purified water to crystallize, filter, and dry to obtain off-white solid 8-chloro- 5,6-Dihydro-11H-benzo[5,6]cycloheptano-[1,2-b]pyridin-11-one, HPLC detection product purity and maximum simple heterogeneity, the results are shown in Table 2.
[0073] The influence of table 2 ...
Embodiment 3
[0079] With the catalyst prepared in Example 1, tetrahydrofuran as the impact of the consumption of the solvent investigation catalyst and the consumption of solvent tetrahydrofuran on the reaction, the preparation process is as follows:
[0080] a) Place 3.4g of raw material amide SM and catalyst (based on the amount of amide SM added) in tetrahydrofuran (ml / g, that is, the number of milliliters of solvent added per gram of substrate raw material SM), and heat up to reflux for reaction;
[0081] b) When HPLC detects that the raw material amide SM no longer decreases, cool down to room temperature and filter to remove the catalyst to obtain a filtrate;
[0082] c) adding 10ml of 2mol / L aqueous sodium hydroxide solution to the filtrate to adjust the pH to 10-11;
[0083] d) Heat up to 80-90°C and stir for 2-3 hours for hydrolysis, add dropwise 2mol / L hydrochloric acid aqueous solution to adjust the pH of the system to 6-7, add dropwise purified water to crystallize, filter, and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com