Preparation method of catalyst used for synthesizing ethyl alcohol through hydrogenation on dimethyl oxalate, catalyst obtained by adopting preparation method and application thereof
A dimethyl oxalate and catalyst technology, applied in the field of catalyst preparation, can solve the problems of restricting large-scale application, easy sintering, and accelerated catalyst deactivation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
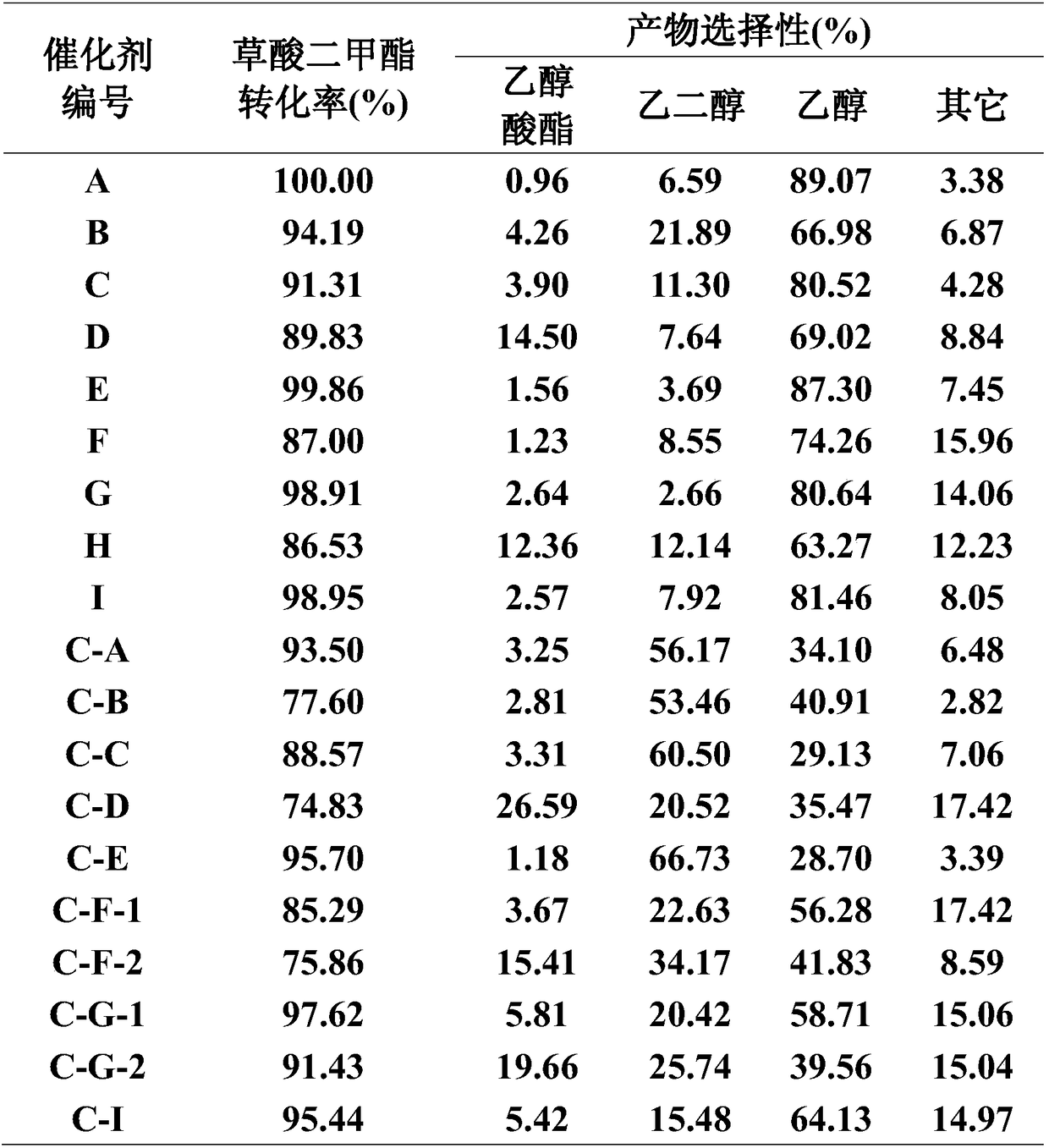
Examples
Embodiment 1
[0071] Catalyst preparation
[0072] Weigh 1.37g of copper nitrate trihydrate and dissolve in 50ml of deionized water to form solution I, weigh 0.28g of boric acid and dissolve in 50ml of deionized water to obtain solution II, and mix solutions I and II to obtain solution III. 2.4 g of urea was added to solution III, and after mixing and stirring for 30 min, 2 g of carbon nanotube carrier was added into the obtained solution, and stirring was continued for 60 min. Then the obtained mixture was put into a hydrothermal synthesis tank, and hydrothermally synthesized at 100° C. for 24 h at a rotational speed of 2 r / min. The obtained hydrothermal synthesis product was filtered, washed with deionized water, placed in a closed autoclave, and continuously fed with supercritical CO 2 , Dry at 40°C and 10MPa for 20h. Then it was calcined at 500° C. for 3 h in a nitrogen atmosphere in a tube furnace to obtain catalyst A, which contained 15% Cu and 2% B in terms of elements.
[0073] C...
Embodiment 2
[0076] Weigh 0.80g of ferric nitrate nonahydrate and dissolve in 50ml of deionized water to form solution I, weigh 0.17g of silver nitrate and dissolve in 50ml of deionized water to obtain solution II, and mix solutions I and II to obtain solution III. Add 6.3g of urea to the solution III, mix and stir for 30min, then add 5g of 40wt% silica sol dropwise, and continue stirring for 120min. Then the obtained mixture was put into a hydrothermal synthesis tank, and hydrothermally synthesized at 120° C. for 10 h at a rotation speed of 2 r / min. The obtained hydrothermal synthesis product was filtered, washed with deionization, placed in a closed autoclave, and continuously fed with supercritical CO 2 , Dry at 40°C and 10MPa for 24h. Then it was calcined at 350° C. for 3 h in an air atmosphere in a tube furnace to obtain catalyst B, which contained 5% Fe and 5% Ag in terms of elements.
[0077] The catalyst reduction and hydrogenation reaction steps in Example 1 were repeated, excep...
Embodiment 3
[0079] Weigh 0.58g of silver nitrate and dissolve it in 50ml of deionized water to form solution I, weigh 0.23g of lanthanum nitrate hexahydrate and dissolve it in 50ml of deionized water to obtain solution II, and mix solutions I and II to obtain solution III. Add 1.6 g of urea to solution III, mix and stir for 30 min, then add 2 g of graphene carrier into the obtained solution, and continue stirring for 60 min. Then the obtained mixture was put into a hydrothermal synthesis tank, and hydrothermally synthesized at 180° C. for 24 hours at a rotational speed of 2 r / min. The obtained hydrothermal synthesis product was filtered, washed with deionized water, placed in a closed autoclave, and continuously fed with supercritical CO 2 , Dry at 40°C and 10MPa for 48h. Then it was calcined at 500° C. for 3 h in a nitrogen atmosphere in a tube furnace to obtain catalyst C, which contained 15% Ag and 3% La in terms of elements.
[0080] The catalyst reduction and hydrogenation reaction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com