Method for highly producing benzene from C6 alkane
An alkane, high-yield technology, used in the purification/separation of hydrocarbons, chemical instruments and methods, including molecular sieve catalysts, etc. focus effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
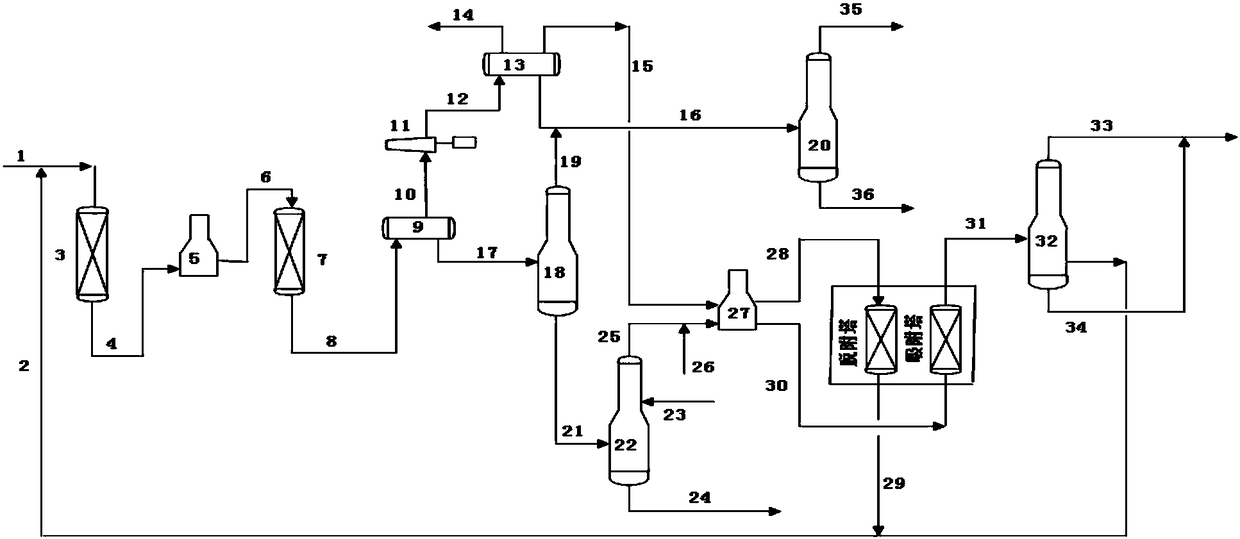
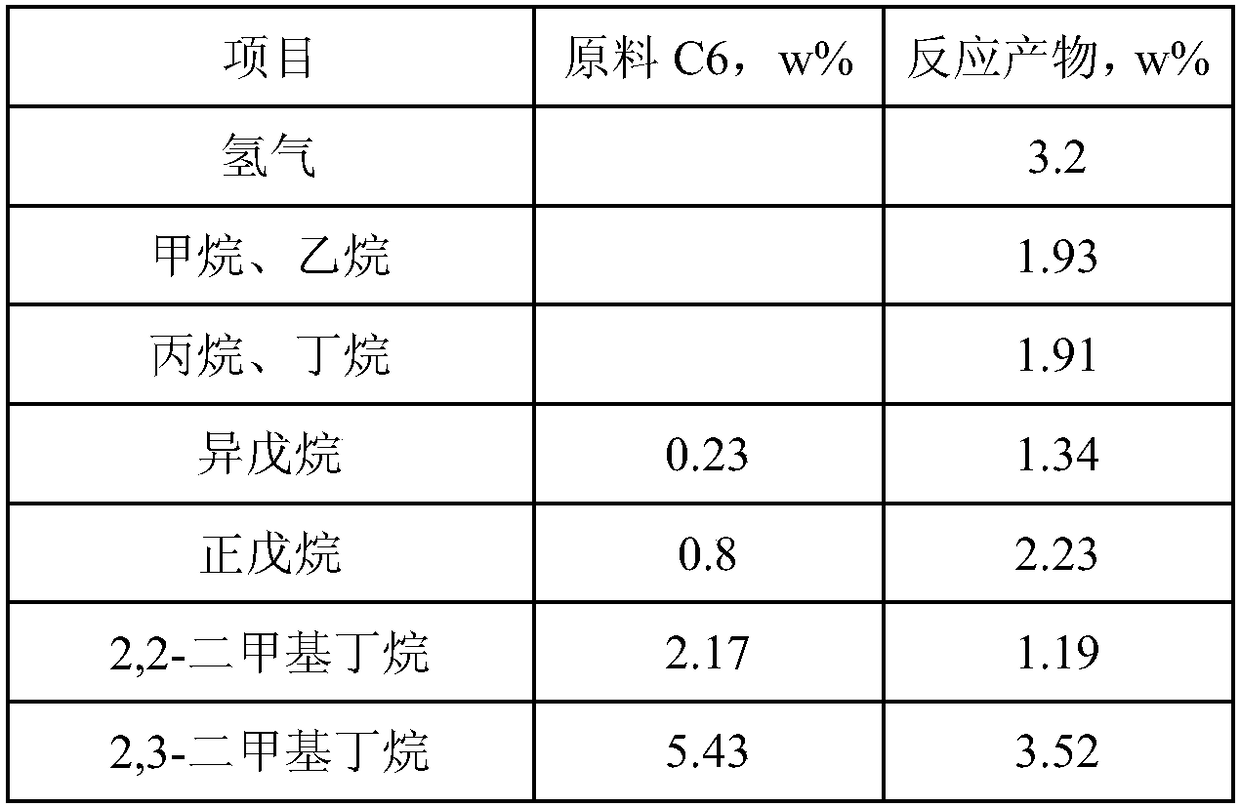
[0055] In this embodiment, the attached figure 1 In all the shown process flow, the C6 raw material is introduced into the above-mentioned reaction and separation system. The composition of alkane C6 feedstock and reaction product is shown in Table 2 below. Reaction conditions for producing more benzene: reaction temperature 480°C, pressure 0.35MPa(g), hydrogen-oil volume ratio 600:1, raw material volume space velocity 2.5h -1. At this time, the conversion rate of methylpentane is about 47.34%, the conversion rate of n-hexane is about 58.78%, and the yield of benzene reaches 41%.
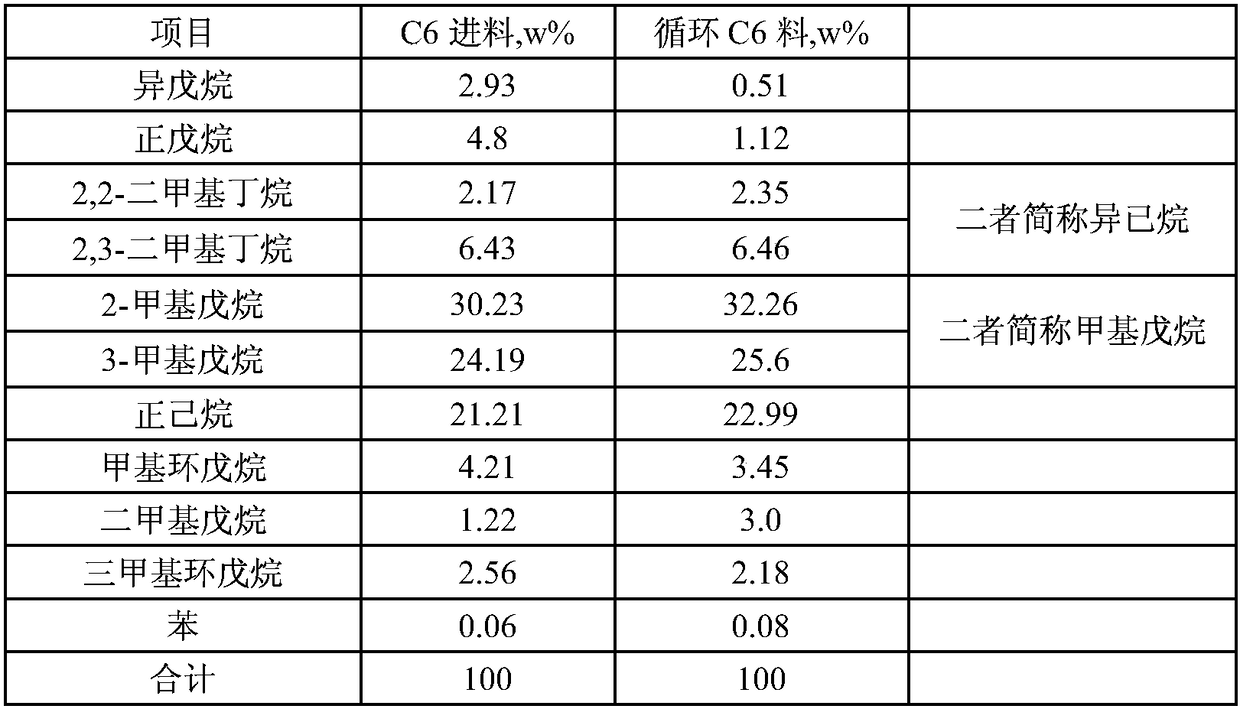
[0056] According to the process of the present invention, the reaction product is separated and the material after benzene is extracted and mixed with fresh C6 feed into the 5A molecular sieve adsorption system and the feed composition of the deisohexane tower after desorbing n-hexane is shown in Table 3. At this time, the adsorption temperature of the 5A molecular sieve system is 290°C, the adso...
Embodiment 2
[0068] In this embodiment, the attached figure 1 For all the shown process flow, the C6 raw material is introduced into the above-mentioned reaction separation system. The composition of alkane C6 feedstock and reaction product is shown in Table 6 below. Reaction conditions for prolific benzene: reaction temperature 490°C, pressure 0.40MPa(g), hydrogen to oil volume ratio 600:1, raw material volume space velocity 2.5h -1 . At this time, the conversion rate of methylpentane is about 48.21%, the conversion rate of n-hexane is about 59.58%, and the yield of benzene reaches 39.67%.
[0069] According to the process of the present invention, the reaction product is separated and the material after benzene is extracted and mixed with fresh C6 feed into the 5A molecular sieve adsorption system and the feed composition of the deisohexane tower after desorbing n-hexane is shown in Table 7. At this time, the adsorption temperature of the 5A molecular sieve system is 300°C, the adsorp...
Embodiment 3
[0080] In this embodiment, the attached figure 1 For all the shown process flow, the C6 raw material is introduced into the above-mentioned reaction separation system. The compositions of alkane C6 feedstock and reaction products are shown in Table 10 below. Reaction conditions for prolific benzene: reaction temperature 500°C, pressure 0.30MPa(g), hydrogen to oil volume ratio 600:1, raw material volume space velocity 2.5h -1 . At this time, the conversion rate of methylpentane is about 47.18%, the conversion rate of n-hexane is about 58.05%, and the yield of benzene reaches 40.67%.
[0081] According to the process of the present invention, the reaction product is separated and the material after benzene is extracted and mixed with fresh C6 feed into the 5A molecular sieve adsorption system and the feed composition of the deisohexane tower after desorbing n-hexane is shown in Table 11. At this time, the adsorption temperature of the 5A molecular sieve system is 310°C, the a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com