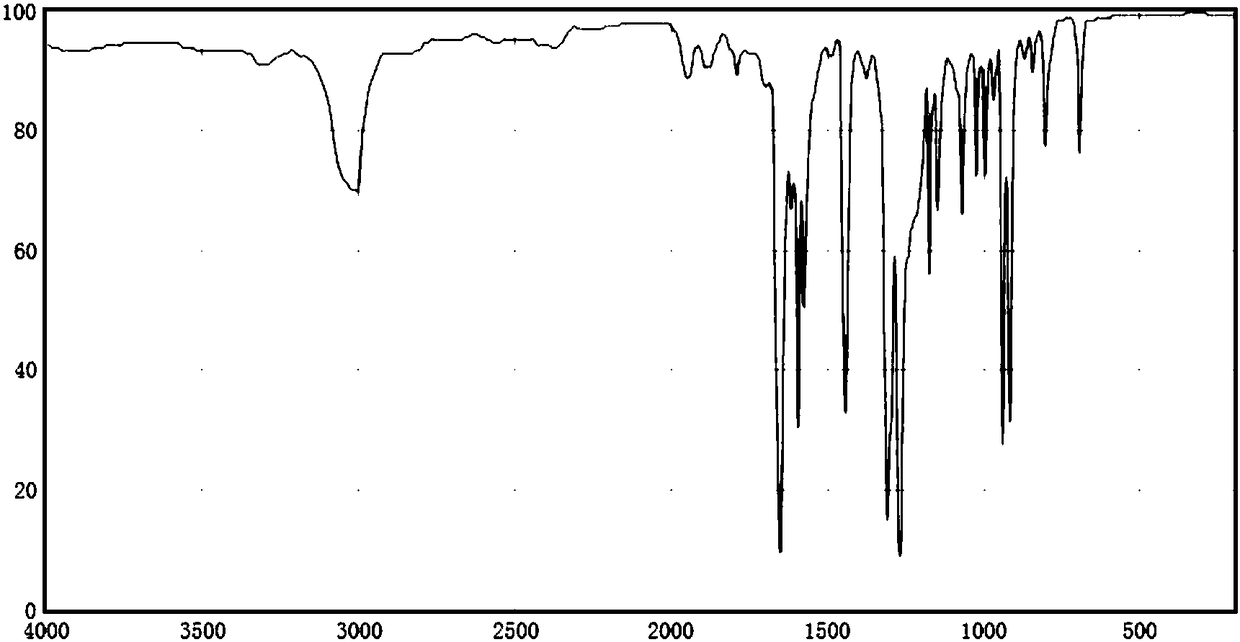
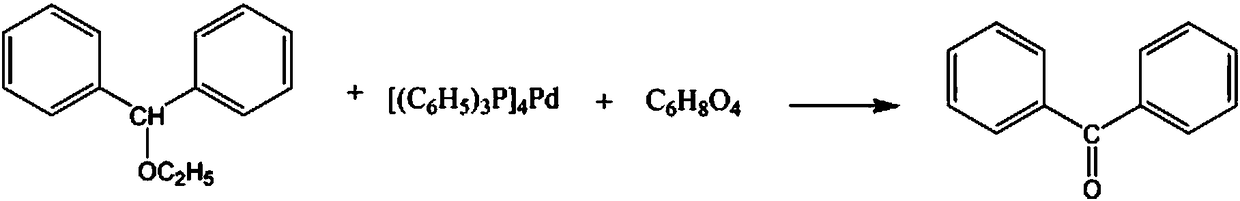Synthetic method for dicycloethyl piperidine drug intermediate benzophenone
A technology of bicycloethylpiperidine and benzophenone, which is applied in the preparation of pharmaceutical intermediates and the field of synthesis of bicycloethylpiperidine drug intermediate benzophenone, which can solve the adverse effects on the personal safety of synthesis operators, equipment manufacturing and Increased maintenance costs, high corrosion resistance requirements, etc., to achieve the effects of reducing pollution treatment costs, increasing reaction yield, and shortening reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The synthetic method of benzophenone, a bicycline drug intermediate, comprises the steps:
[0018] A: Add 3mol benzhydryl ethyl ether into the reaction vessel, raise the temperature to 40°C, add 900ml of sodium sulfate solution with a mass fraction of 15% in 2 times within 60min, continue to stir, react for 2h, and then add 3mol tetrakis(triphenylphosphine)palladium powder, gradually increase the temperature, and rise to 55°C within 30min;
[0019] B: Then add 6 mol mass fraction of dimethyl fumarate solution with a mass fraction of 30%, control the stirring speed at 330rpm, continue the reaction for 90min, lower the temperature to 20°C, separate the layers of the solution, separate the oil layer, and the mass fraction is 10% Potassium chloride solution was washed 4 times, refluxed 3 times with 40% dibutylamine solution, 2 times with 50% diethylene glycol monobutyl ether solution, 70% 1,3- Recrystallize in dichloropropene solution and dehydrate with activated alumina d...
Embodiment 2
[0021] The synthetic method of benzophenone, a bicycline drug intermediate, comprises the steps:
[0022] A: Add 3mol benzhydryl ethyl ether to the reaction vessel, increase the temperature to 45°C, add 900ml of sodium sulfate solution with a mass fraction of 17% in 3 times within 75min, continue to stir, react for 2.5h, and then Add 3.5mol tetrakis(triphenylphosphine) palladium powder, gradually increase the temperature, and raise it to 57°C within 40min;
[0023] B: Then add 7mol mass fraction of dimethyl fumarate solution with a mass fraction of 33%, control the stirring speed at 345rpm, continue the reaction for 110min, lower the temperature to 22.5°C, separate the layers of the solution, separate the oil layer, and the mass fraction is 13% Potassium chloride solution was washed 5 times, refluxed 4 times with 42.5% dibutylamine solution, 3 times with 53% diethylene glycol monobutyl ether solution, 73% 1,3- Recrystallized in dichloropropene solution and dehydrated with anh...
Embodiment 3
[0025] The synthetic method of benzophenone, a bicycline drug intermediate, comprises the steps:
[0026] A: Add 3mol benzhydryl ethyl ether into the reaction vessel, raise the temperature to 47°C, add 900ml sodium sulfate solution with a mass fraction of 22% in 3 times within 90min, continue to stir, react for 3h, then add 4mol tetrakis(triphenylphosphine)palladium powder, gradually increase the temperature to 60°C within 50min;
[0027] B: Then add 8mol mass fraction of 37% dimethyl fumarate solution, control the stirring speed at 360rpm, continue the reaction for 130min, lower the temperature to 25°C, separate the layers of the solution, separate the oil layer, and the mass fraction is 16% Wash 5 times with potassium chloride solution, reflux 4 times with 45% dibutylamine solution, 3 times with 57% diethylene glycol monobutyl ether solution, 76% 1,3- Recrystallize in dichloropropene solution and dehydrate with activated alumina dehydrating agent to obtain 535.08 g of finis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com