Transdermally delivered ursolic acid/insulin nanoscale slow-release preparation and preparation method thereof
A nano-sustained-release insulin technology, applied in the field of pharmacy, can solve the problems of low absorption and bioavailability, poor water solubility of insulin, etc., and achieve the effect of reducing the number of medications, reducing the number of medications, and improving solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A: Dissolve 1mg of insulin in 1ml of methanol to form a drug-containing organic solution, dissolve 9.06mg of ursolic acid in methanol, 0.1ml of glycerin, 200mg of hydroxymethylcellulose, 1g of mannitol, 0.4g of propylene glycol, and carbopol Sodium acrylate 5mg, sodium acrylate 150mg, dissolved in water to form a hydrophilic aqueous solution;
[0042] B: Mix 100 μL insulin methanol solution and 100 μL ursolic acid methanol solution evenly, add dropwise to the hydrophilic aqueous solution, and stir at room temperature for 1 hour to obtain ursolic acid / insulin nanoparticles.
[0043]C: Dissolve glycerin, hydroxymethylcellulose, mannitol, propylene glycol, carbomer, and sodium acrylate in 3ml of water as an aqueous solution of hydrophilic excipients, freeze-dry ursolic acid / insulin nanoparticles and mix with the aqueous solution of hydrophilic excipients Mix evenly to obtain the drug-loaded layer with sustained release function.
[0044] D: Apply the drug-loaded layer eve...
Embodiment 2
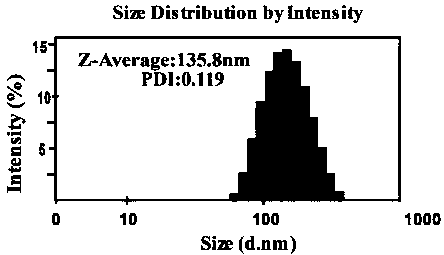
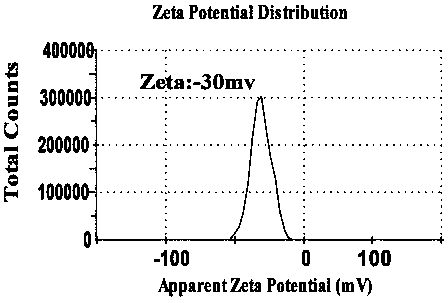
[0046] The ursolic acid / insulin nanometer prepared by the present invention is measured average particle diameter, PDI and electric potential by dynamic light scattering, as figure 1 and figure 2 shown.
Embodiment 3
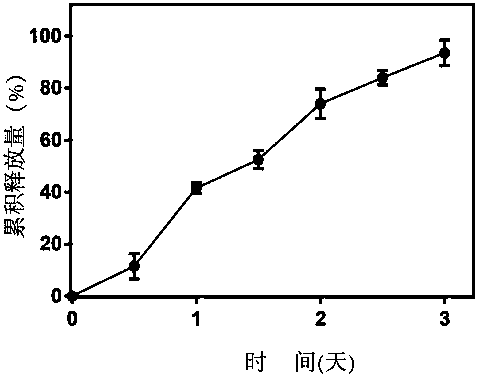
[0048] Put 1ml of the drug-loaded layer in a dialysis bag with a molecular weight cut-off of 10,000, and at the same time place the dialysis bag in 30ml of PBS phosphate buffer solution with a pH of 7.4, then put the PBS buffer solution in a shaker at a constant temperature at 37°C, and the speed 233r / min, sample 1ml at different time points, and add 1ml of blank PBS at the same time. Adopt ultraviolet spectrophotometry to detect wavelength 276nm, calculate cumulative amount, such as image 3 Shown, show that the ursolic acid / insulin nano sustained-release transdermal drug preparation prepared by the present invention has the characteristics of sustained release.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com