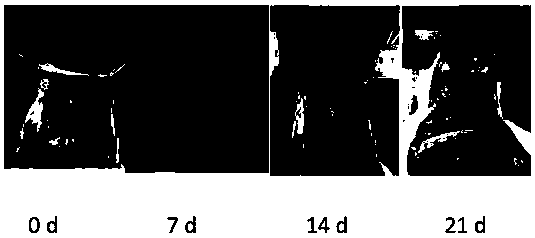
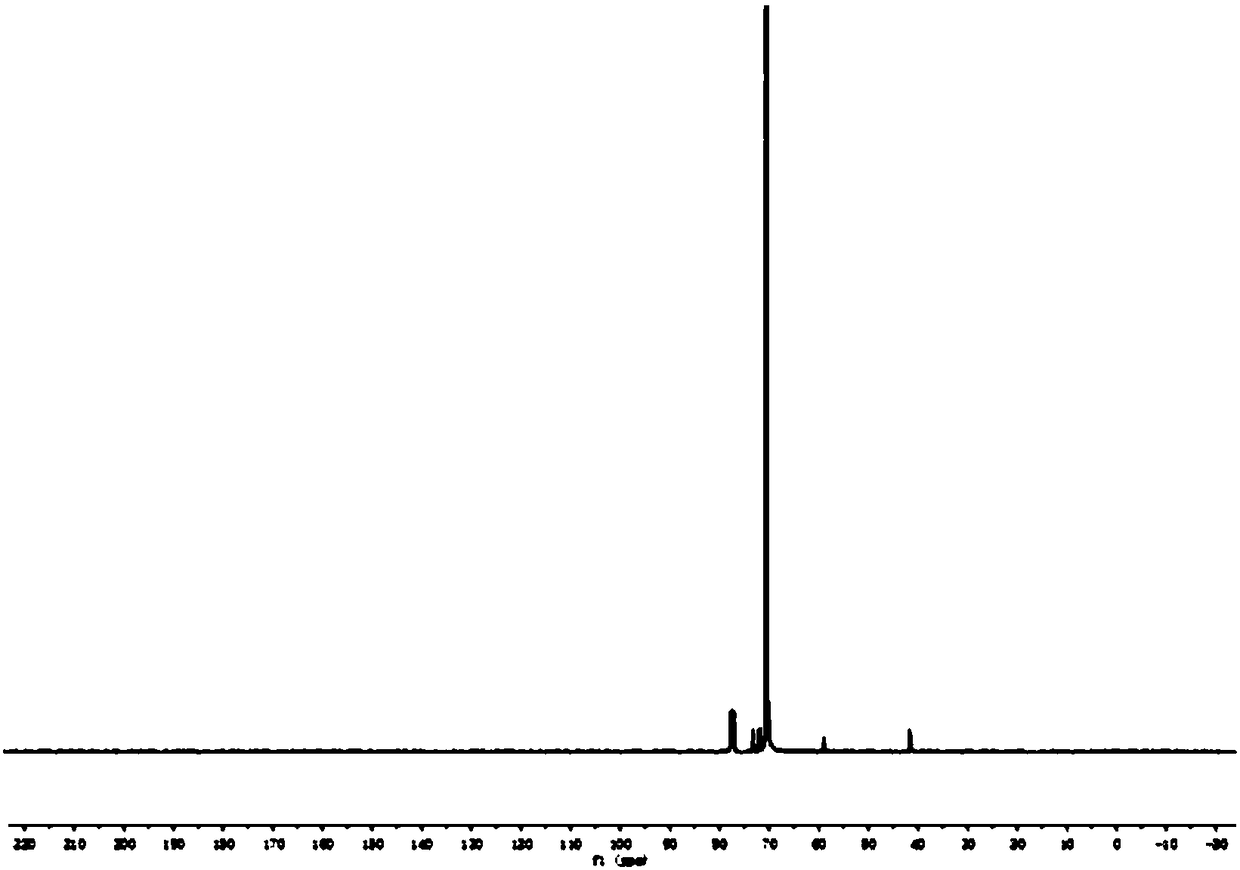
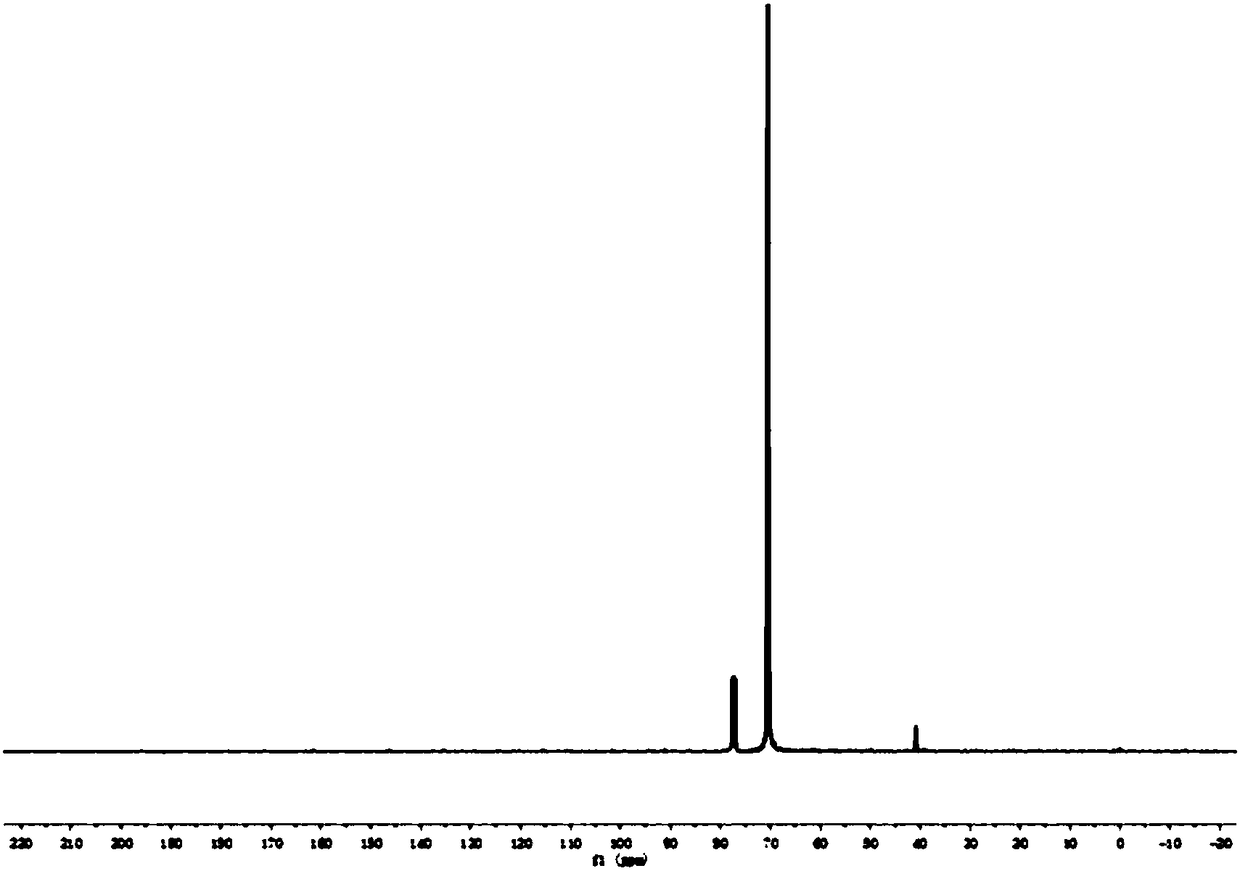
Polyamino acid, preparation method thereof and drug-loaded gel
A polyamino acid and amino acid technology, applied in the field of polymers, can solve the problems of poor water solubility, fast metabolism, and low drug utilization, and achieve good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] The present invention provides a method for preparing polyamino acid described in formula (I), comprising:
[0046] Reaction of amino-terminated polyethylene glycol monomethyl ether with the structure of formula (III) and amino acid-N-internal carboxylic acid anhydride in a solvent to obtain polyamino acid; the amino acid-N-internal carboxylic acid anhydride is glycine-N-internal carboxylic acid anhydride anhydride, valine-N-endocarboxylic anhydride, leucine-N-endocarboxylic anhydride or alanine-N-endocarboxylic anhydride;
[0047]
[0048] Among them, -R 1 -H and -R 2 for -H,
[0049] or, -R 1 for -CH 3 and-R 2 for -CH 3 ;
[0050] 50≤i≤200, 3≤j≤30;
[0051] In formula (III), 50≤i≤200.
[0052] In an organic solvent, the amino-terminated polyethylene glycol monomethyl ether is respectively mixed with glycine-N-endocarboxylic anhydride, valine-N-endocarboxylic anhydride, alanine-N-endocarboxylic anhydride, leucine-N - Internal carboxylic acid anhydride re...
Embodiment 1
[0110] Put 20g of polyethylene glycol monomethyl ether with a number average molecular weight of 2000 into a dry reaction bottle, then vacuumize it at 120°C for 2 hours while stirring, put the reaction bottle in an oil bath, and when the temperature is 60°C 10g of anhydrous magnesium sulfate and 200mL of dichloromethane were added to the reaction flask respectively, and 5mL of triethylamine and 30mL of methanesulfonyl chloride were added to the reaction flask in an ice-water bath. React for 72 hours under the condition of agitator stirring. After the reaction, the obtained reaction product was washed five times with saturated sodium chloride solution, and then dried overnight by adding anhydrous magnesium sulfate; After drying under vacuum for 24h, an intermediate product was obtained;
[0111]Dissolve the intermediate product and ammonium chloride with the same quality as the intermediate product in ammonia water, the volume of ammonia water is 10 times the mass of the interm...
Embodiment 2
[0119] Put 20g of polyethylene glycol monomethyl ether with a number-average molecular weight of 4000 into a dry reaction bottle, then vacuumize it at 120°C for 2 hours while stirring, put the reaction bottle in an oil bath, and when the temperature is 60°C , add 10g of anhydrous magnesium sulfate, 200mL of dichloromethane dehydrated into the reaction flask respectively, add 2.5mL of triethylamine and 15mL of methanesulfonyl chloride into the reaction flask under the condition of ice-water bath, at 25°C 1. Reaction 72h under stirring bar stirring condition, after the reaction finishes, the reaction product obtained is washed five times with saturated sodium chloride solution and then dried overnight by adding anhydrous magnesium sulfate; the product after drying is settled with anhydrous ether, and the After vacuum drying at ℃ for 24h, an intermediate product was obtained;
[0120] Dissolve the intermediate product and ammonium chloride with the same quality as the intermediat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com