Nasal nano-preparation mometasone furoate liquid crystal gel nanoparticle and preparation method thereof
A technology of mometasone furoate and nano-preparation, which is used in anti-inflammatory agents, liquid delivery, pharmaceutical formulations, etc., can solve the problem that the moisturizing and moisturizing effect does not reach the best level, the particle size of liquid crystal nanoparticles is not suitable, and the molecular weight of furoate is not suitable. The problem of low content of metasone, etc., can achieve the effect of maintaining the relative stability of particle size, avoiding the risk of hemolysis, and improving the sustained release time and absorption rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
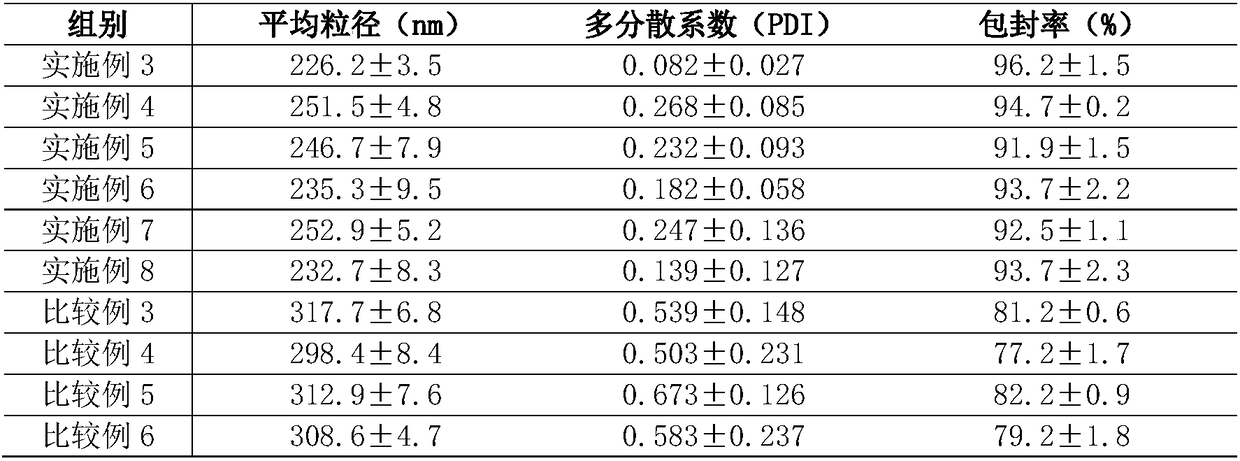
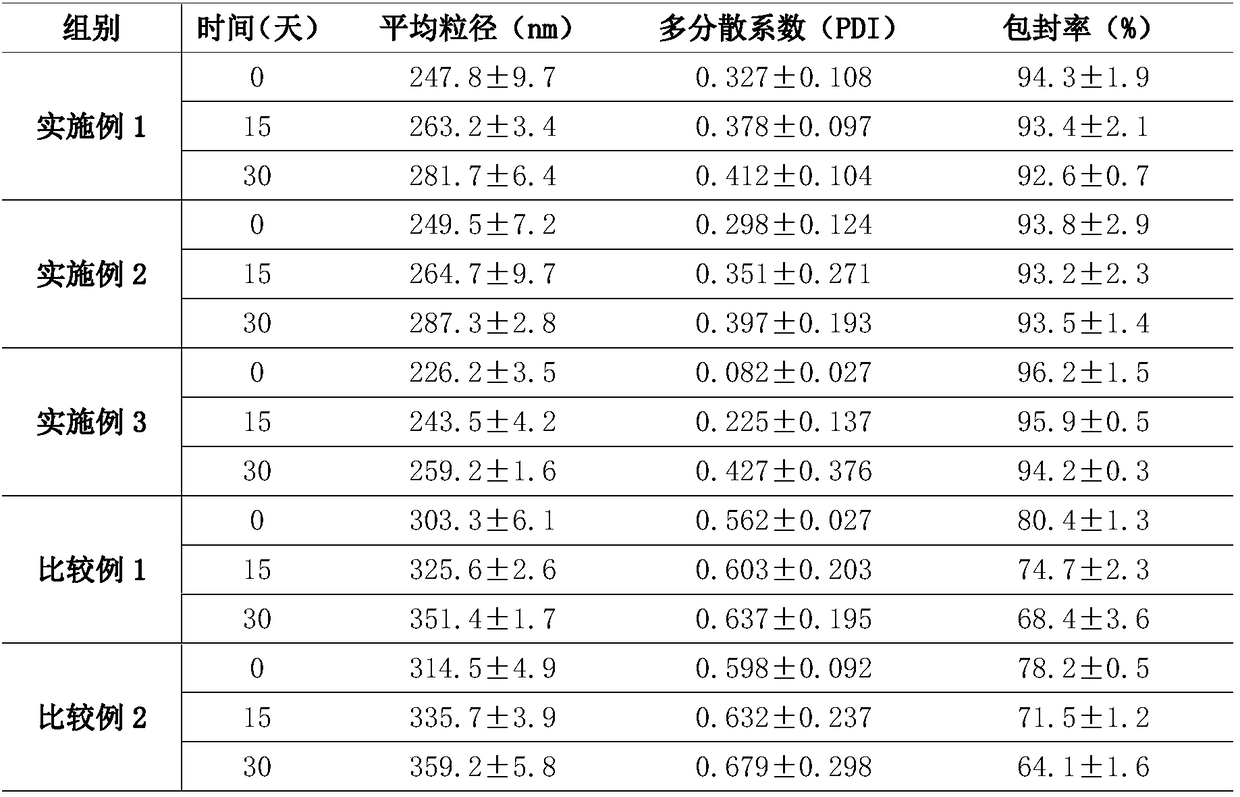
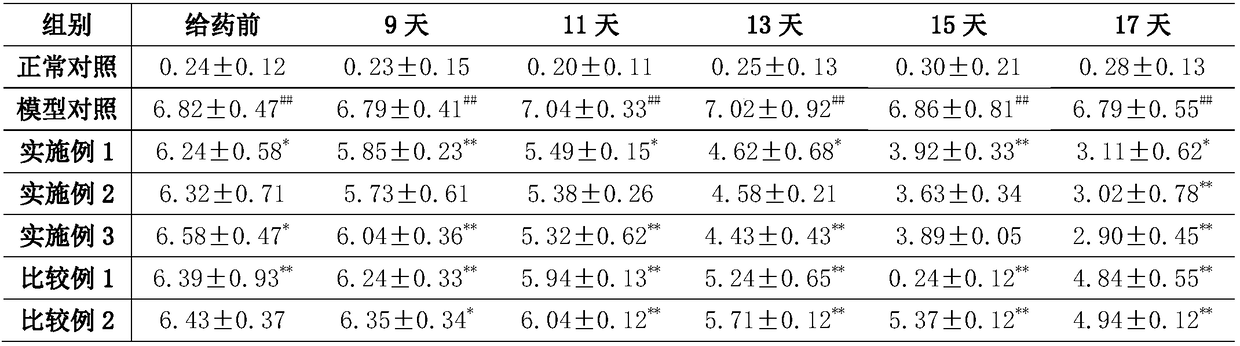
[0025] This embodiment provides a nasal cavity nano-preparation mometasone furoate liquid crystal gel nanoparticles, including raw materials: mometasone furoate 0.09wt%, diacylglycerol 50wt%, soybean lecithin 20wt%, polysorbate-80 5wt%, absolute ethanol 5wt%, deionized water 19.91wt%.
[0026] The preparation method of the nasal cavity nano-preparation mometasone furoate liquid crystal gel nanoparticles provided in this embodiment comprises the following steps:
[0027] S1. Weigh mometasone furoate, diacylglycerol, soybean lecithin, polysorbate-80, heat and stir at 30-45°C to obtain a mixed solution;
[0028] S2. Add anhydrous solvent to the mixed solution obtained in S1, and perform ultrasonic dispersion for 3 to 5 minutes to obtain a clear and transparent liquid crystal gel nanoparticle precursor solution;
[0029] S3. Add deionized water to 100wt% in the liquid crystal nanoparticle gel precursor solution obtained in S2, and then further disperse through a high-shear homoge...
Embodiment 2
[0031] This embodiment is basically the same as Embodiment 1, the difference lies in the following aspects:
[0032] A nasal cavity nano-preparation mometasone furoate liquid crystal gel nanoparticles, comprising raw materials: mometasone furoate 0.06wt%, diacylglycerol 30wt%, soybean lecithin 30wt%, polysorbate-80 15wt%, anhydrous Ethanol 15wt%, deionized water 9.94wt%.
Embodiment 3
[0034] This embodiment is basically the same as Embodiment 1, the difference lies in the following aspects:
[0035] A nasal cavity nano-preparation mometasone furoate liquid crystal gel nanoparticles, comprising raw materials: mometasone furoate 0.08wt%, diacylglycerol 40wt%, soybean lecithin 25wt%, polysorbate-80 10wt%, anhydrous Ethanol 10wt%, deionized water 14.92wt%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com