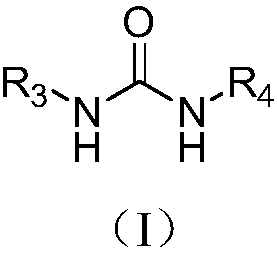
Synthesis method of biaryl compounds
A synthesis method and technology of aromatic compounds, which are applied in the preparation of organic compounds, carbon compound catalysts, chemical instruments and methods, etc., can solve the problems of unavailable catalysts, low reaction yields, and poor selectivity, and achieve green, safe, and safe synthesis reactions. The effect of shortening the reaction time and improving the synthesis efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] (1) Put 10mol% of diethylurea (0.02mmol), 0.6mmol of potassium tert-butoxide, 0.2mmol of p-iodoanisole and 2ml of benzene in a reaction tube, heat and stir for reaction, and the heating and stirring temperature is 120°C , The reaction time is 24h;.
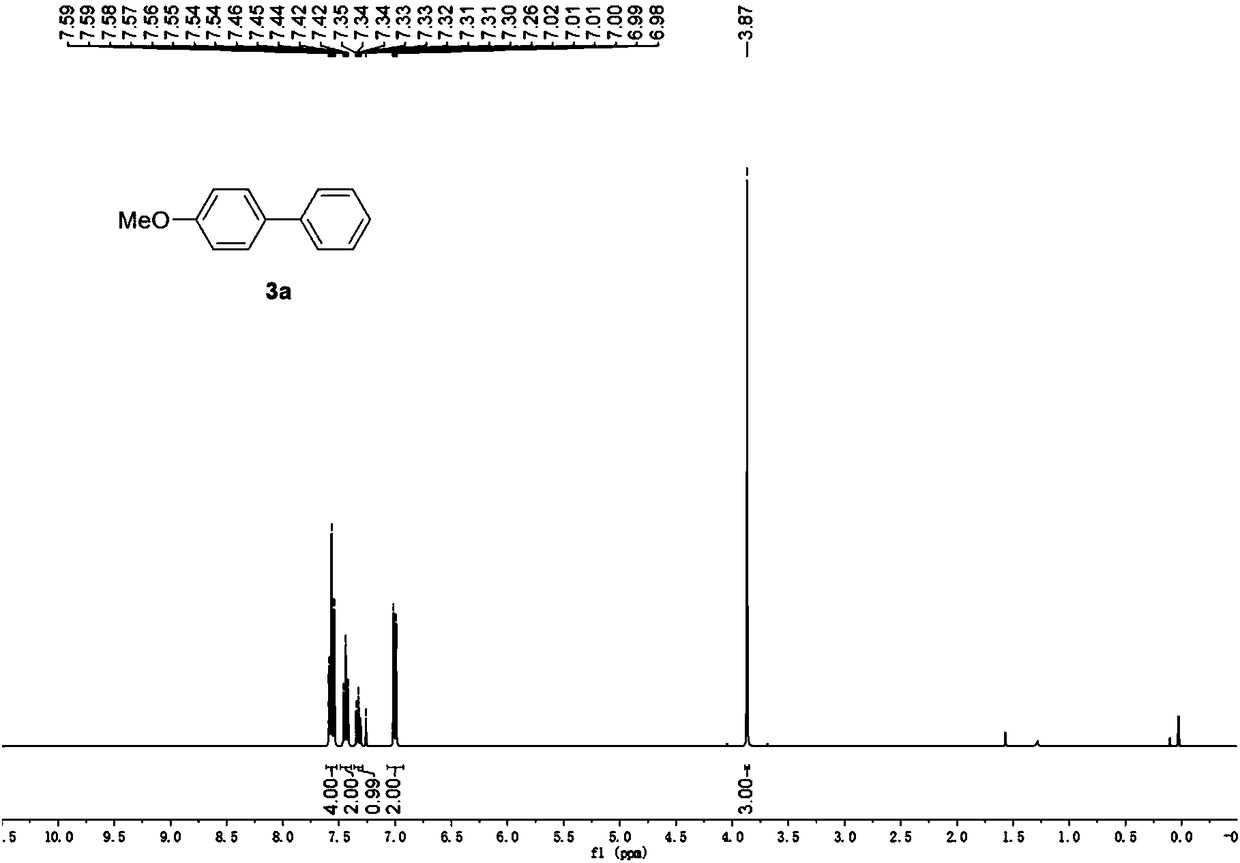
[0049] (2) After the reaction is over, separate by column chromatography (the chromatographic column filler is 300-400 mesh column chromatography silica gel, and the eluent is: petroleum ether: ethyl acetate=100:0.5, v:v), the product can be obtained 4-Methoxybiphenyl, yield 94%. The structural characterization of the product is as figure 1 shown.
[0050] The structural characterization data of the product are as follows:
[0051] 1 H NMR (400MHz, CDCl 3 )δ7.59–7.54(m,4H),7.44(t,J=7.4Hz,2H),7.32(t,J=7.36Hz,1H),7.00(t,J=8.84Hz,2H),3.87( s,3H).MS(CI) Calcd for C 13 h 12 O:184; Found(M+H + ):185.
Embodiment 2
[0053] (1) Put 10mol% of diethylurea (0.02mmol), 0.6mmol of potassium tert-butoxide, 0.2mmol of m-iodoanisole and 2ml of benzene in a reaction tube, heat and stir for reaction, and the heating and stirring temperature is 120°C , The reaction time is 24h.
[0054] (2) After the reaction is over, separate by column chromatography (the chromatographic column filler is 300-400 mesh column chromatography silica gel, and the eluent is: petroleum ether: ethyl acetate=100:0.5, v:v), the product can be obtained 3-Methoxybiphenyl, yield 51%.
[0055] The structural characterization data of the product are as follows:
[0056] 1 H NMR (400MHz, CDCl 3 )δ7.64–7.61(m,2H),7.47(t,J=7.24Hz,2H),7.41–7.37(m,2H),7.23–7.16(m,2H),6.95–6.92(m,1H) ,3.89(s,3H).MS(CI) Calcd for C 13 h 12 O:184; Found(M+H + ):185.
Embodiment 3
[0058] (1) Put 10mol% of diethylurea (0.02mmol), 0.6mmol of potassium tert-butoxide, 0.2mmol of o-iodoanisole and 2ml of benzene in a reaction tube, heat and stir for reaction, and the heating and stirring temperature is 120°C , The reaction time is 24h.
[0059] (2) After the reaction is over, separate by column chromatography (the chromatographic column filler is 300-400 mesh column chromatography silica gel, and the eluent is: petroleum ether: ethyl acetate=100:0.5, v:v), the product can be obtained 2-Methoxybiphenyl, the yield was 82%.
[0060] The structural characterization data of the product are as follows:
[0061] 1 H NMR (400MHz, CDCl 3 )δ7.57–7.54(m,2H),7.45–7.41(m,2H),7.36–7.33(m,3H),7.08–7.00(m,2H),3.83(s,3H).MS(CI) Calcd for C 13 h 12 O:184; Found(M+H + ):185.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com