Application of artemisinin and derivative thereof in preparation of drug for preventing and treating porcine reproductive and respiratory syndrome
A technology for respiratory syndrome and derivatives, which is applied in the direction of pharmaceutical formulas, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problems of unsatisfactory vaccine prevention and control effects, inability to stimulate cellular immunity, and long-term effects, and achieve drug Effective components and mechanism of action are clear, and the preparation process is mature and reliable.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
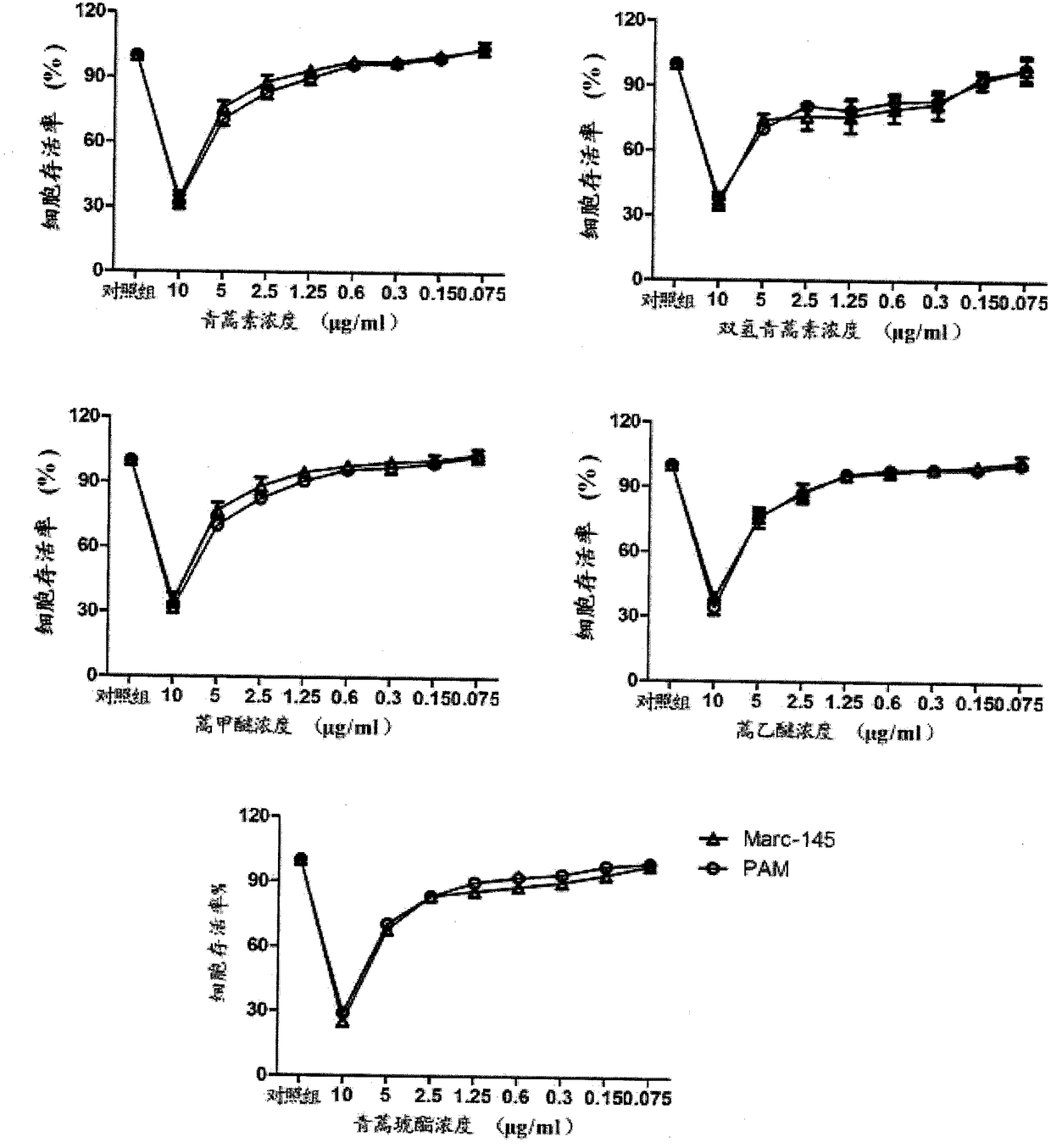
[0024] Example 1: Effects of artemisinin and its derivatives on the viability of Marc-145 cells and PAM cells
[0025] After inoculating Marc-145 cells or PAM cells in a 96-well cell culture plate and growing to a monolayer, the medium was discarded, washed twice with PBS (phosphate buffered saline), and the drug was treated with a cell maintenance solution containing 2% fetal bovine serum. Dilute to the concentration to be tested, add 100 μL / well, and make three replicate wells for each concentration. After continuing to incubate at 37°C for 48h, add 20μL of 5mg / ml MTT reagent to each well, incubate at 37°C for 4h, discard the supernatant, add 150mL DMSO, shake for 10min to fully dissolve the formed formazan crystals, and detect at 490nm wavelength absorbance value. from figure 1 It can be seen that at the concentration of 2.5 μg / mL of artemisinin and its derivatives, the survival rate of Marc-145 cells and PAM cells is more than 80%, and the growth of cells is not affected...
Embodiment 2
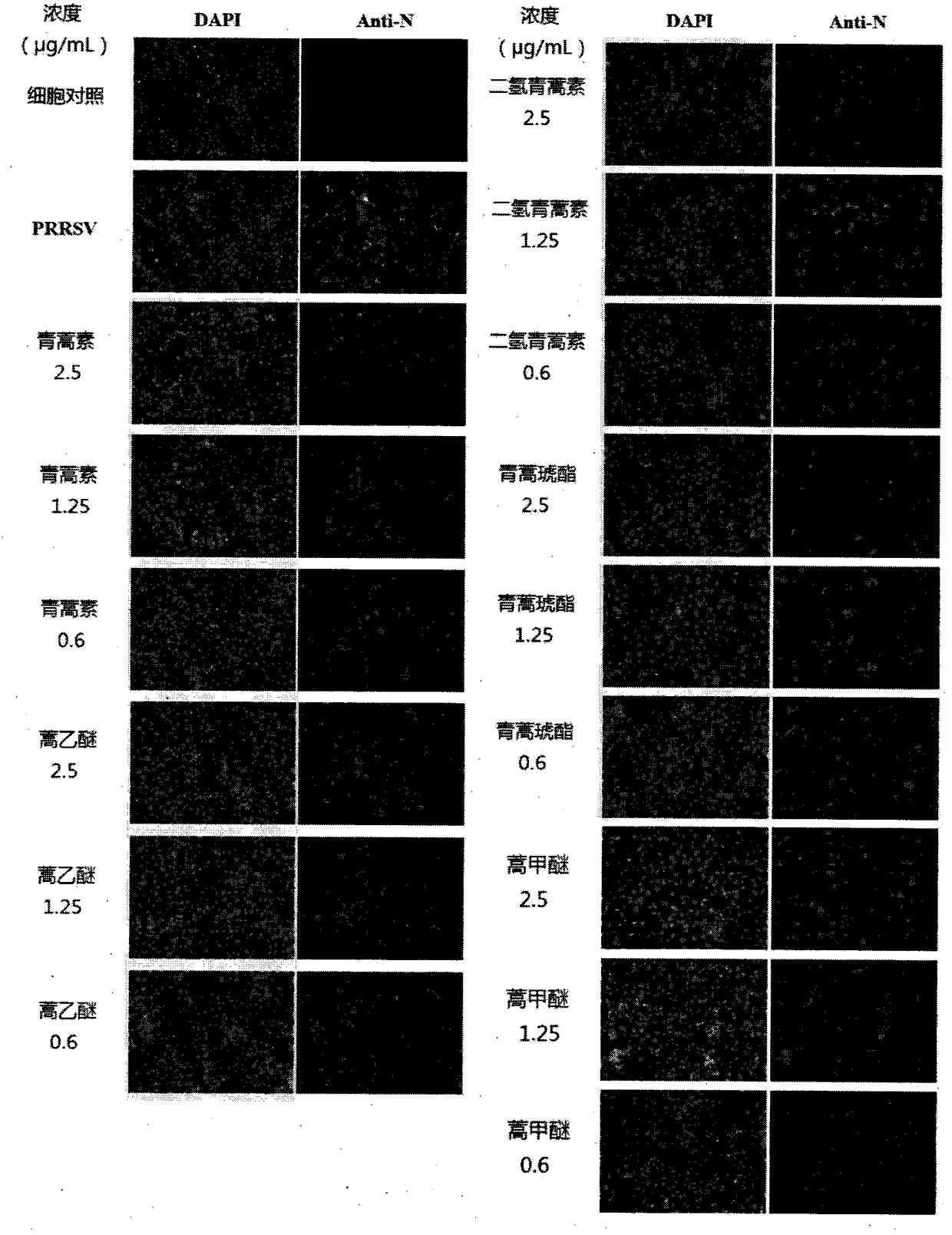
[0026] Example 2: Indirect immunofluorescence assay (IFA) analysis of the inhibition of PRRSV virus N protein synthesis after treatment of Marc-145 cells with different concentrations of artemisinin and its derivatives
[0027] After the Marc-145 cells were seeded in a 6-well cell culture plate and grown to a monolayer, the medium was discarded, washed twice with PBS (phosphate buffered saline), and diluted with DMEM cell maintenance solution containing 2% fetal bovine serum. with 100 TCID 50 of porcine reproductive and respiratory syndrome virus (PRRSV), 1 mL / well, incubated at 37°C for 2 h, discarded the virus supernatant, washed twice with PBS, and added 2.5, 1.25, and 0.6 μg / mL of drugs, 2 mL / well. In the experiment, the normal control group (without the test drug and PRRSV) and the PRRSV control group (without the test drug) were set at the same time, and three parallels were set for each test concentration. The culture was terminated after continuing to culture at 37°C ...
Embodiment 3
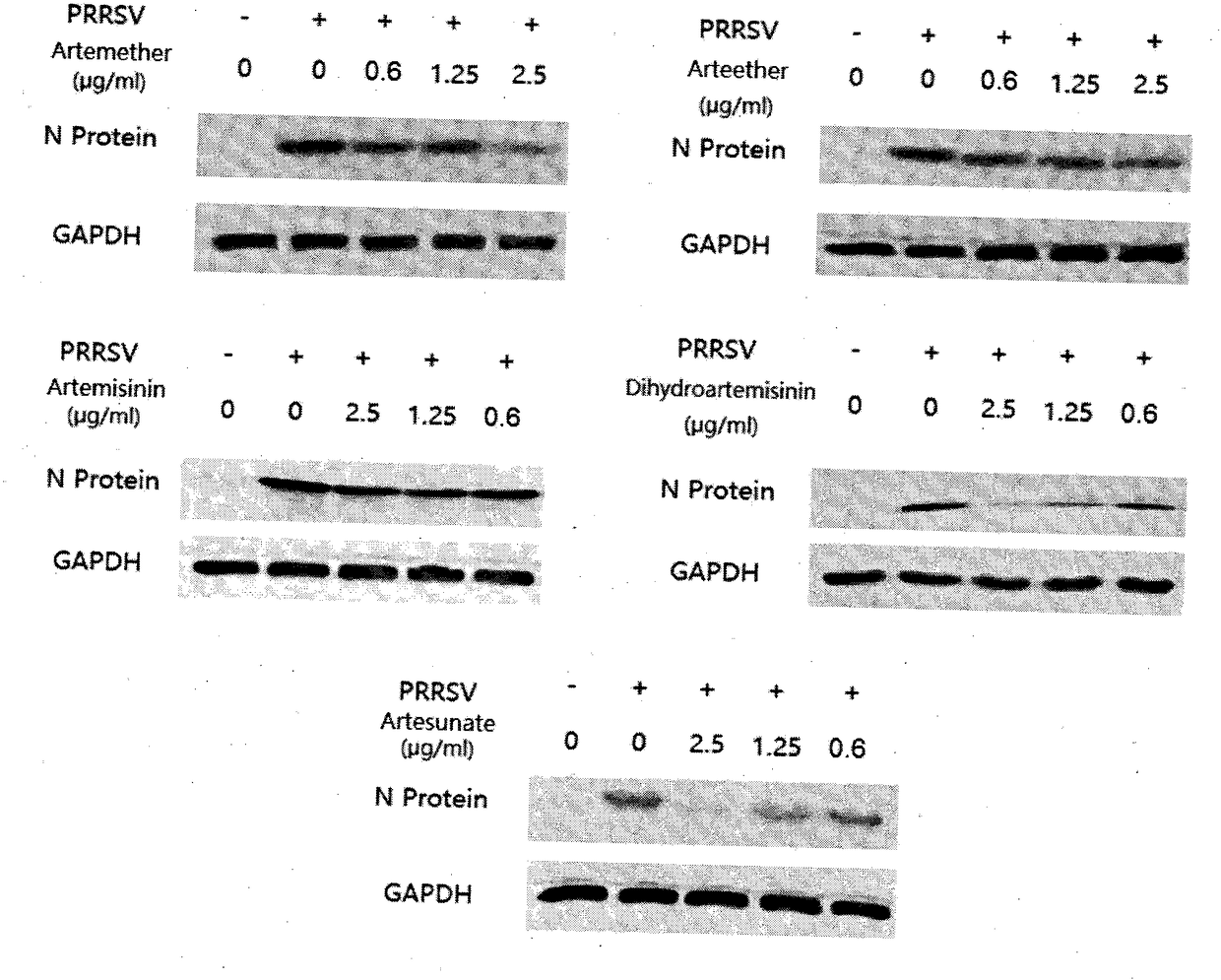
[0028] Example 3: Western Blot analysis of changes in PRRSV virus N protein expression after treatment of Marc-145 cells with different concentrations of artemisinin and its derivatives
[0029] The steps of infecting Marc-145 cells with PRRSV and adding drugs are the same as in step 2. After the cells are cultured at 37° C. for 48 h, the supernatant is discarded and washed twice with PBS. Place the cell culture plate on ice, add 150 μL / well of RIPA lysis solution, and after lysing by pipetting, aspirate the liquid into a centrifuge tube, centrifuge at 12,000 rpm for 5 minutes, and aspirate the supernatant into another clean tube for use. The protein concentration of each sample was determined according to the method of the BCA protein concentration assay kit. The bands of N protein of PRRSV and internal reference protein GAPDH were detected by Western Blot. The test results are as image 3 The results showed that artemisinin and its derivatives had different degrees of inhi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com