Assembling type cell-derived extracellular matrix membrane compound bone repairing material as well as preparation method and application thereof
An acellular matrix and extracellular matrix technology, which is applied in the field of assembled cell-derived extracellular matrix membrane composite bone repair material and its preparation, can solve the problem of difficult standardized production of classical bone tissue engineering products, no systematic report, stem cell tumorigenicity and other problems, to achieve the effects of good osteoinductive ability, enhanced bone regeneration ability, and rapid assembly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
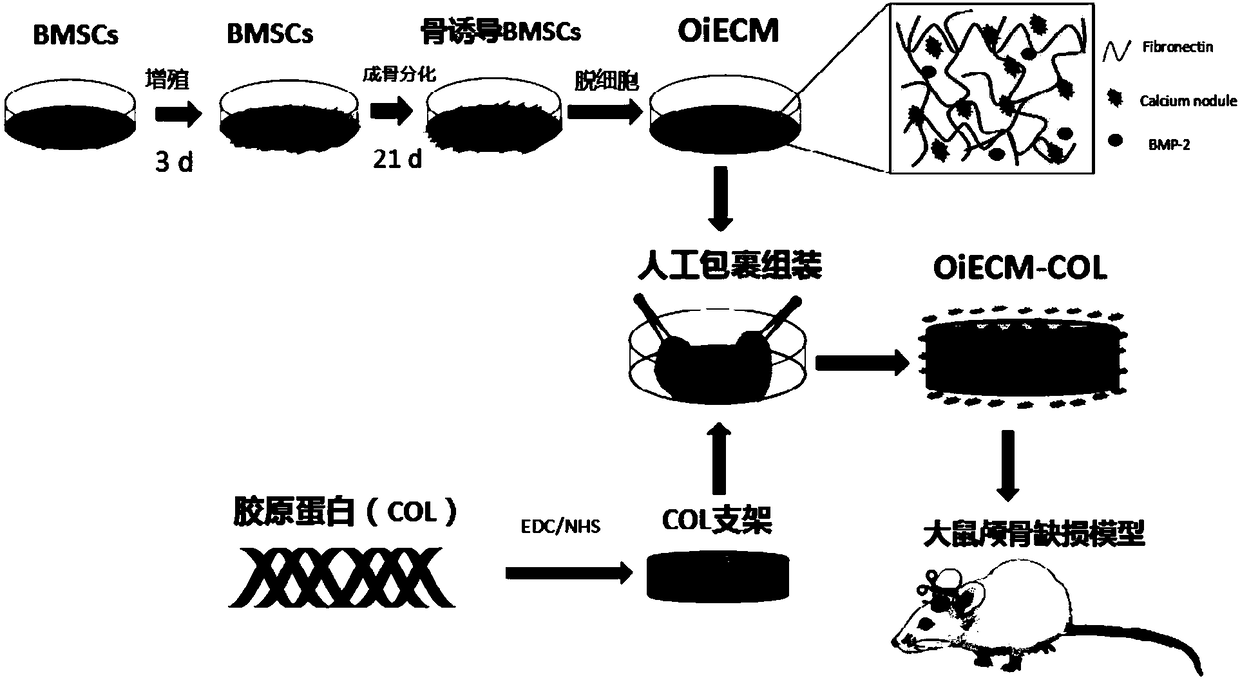
[0048] Embodiment 1: Preparation of a kind of rat osteoinductive acellular matrix membrane wrapped collagen composite bone repair material (OiECM-COL composite bone repair material), its specific preparation process is as follows figure 1 As shown, the detailed steps are as follows:
[0049] (1) Preparation of osteoinductive decellularized matrix membrane (OiECM membrane):
[0050] a. Rat bone marrow mesenchymal stem cells (rat BMSCs, Cat. No. RAWMX-01001, Cyagen Bioscience) were planted in a petri dish, and osteogenic induction medium was added (medium formula: 90% DMEM, 10% calf serum, Penicillin 100U / mL, streptomycin 100μg / mL, L-ascorbic acid 50ug / mL, 10mmol / L β-glycerol phosphate, 100nmol / L dexamethasone, change the solution every three days, at a temperature of 37°C, CO 2 Continuous culture in an incubator with a level of 5% and a pH value of 7.3 for 21 days until the extracellular matrix is secreted and covered the bottom of the dish;
[0051] b. Discard the culture ...
example 2
[0061] Example 2: Acellular Matrix Membrane Encapsulation of Adipose Stem Cells Preparation of composite bone repair materials, the three-dimensional porous collagen scaffold material is selected from DePuy company Its specific preparation process is as follows:
[0062] (1) Preparation of adipose-derived stem cell acellular matrix membrane (adECM membrane):
[0063] a. Rat adipose-derived stem cells (Wistar rat adipose-derived mesenchymal stem cells, Cat. No. RAWMD-01001, Cyagen Bioscience) were planted in a petri dish, and osteogenic induction medium (90% DMEM, calf 10% calf serum, Penicillin 100U / mL, streptomycin 100μg / mL, L-ascorbic acid 50ug / mL, 10mmol / Lβ-glycerol / L phosphate, 100nmol / L dexamethasone), change the medium once every three days, at a temperature of 37°C, CO 2 Continuous culture in an incubator with a level of 5% and a pH value of 7.3 for 21 days until the extracellular matrix is secreted and covered the bottom of the dish;
[0064] b. Remove the cultu...
example 3
[0067] Example 3: Preparation of fibroblast acellular matrix membrane-wrapped chitosan composite bone repair material, the specific steps are as follows:
[0068] (1) Preparation of fibroblast decellularized matrix membrane (fbECM membrane):
[0069] a. Human fibroblasts (human fibroblasts, product number HXXFB-00001, Cyagen Bioscience) were planted in a petri dish, and conditioned medium (90% DMEM, 10% calf serum, penicillin 100U / mL, streptomycin 100μg / mL L-ascorbic acid, 20-100ug / mL L-ascorbic acid), change the solution every three days, at a temperature of 37°C, CO 2 Continuous culture in an incubator with a level of 5% and a pH value of 7.3 for 14 days until the extracellular matrix is secreted and covered the bottom of the dish;
[0070] b. Remove the culture medium from the cell culture dish in step a and gently rinse it with PBS solution for 3 times, add 0.1% SDS to the culture dish and treat it at 37°C for 60 minutes;
[0071] c. Rinse the cell culture dish with PB...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com