Human wnt5a-nl nucleic acid recombinant and its preparation method and application
A recombinant and nucleic acid technology, applied in the field of biomedicine, can solve problems such as bone destruction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
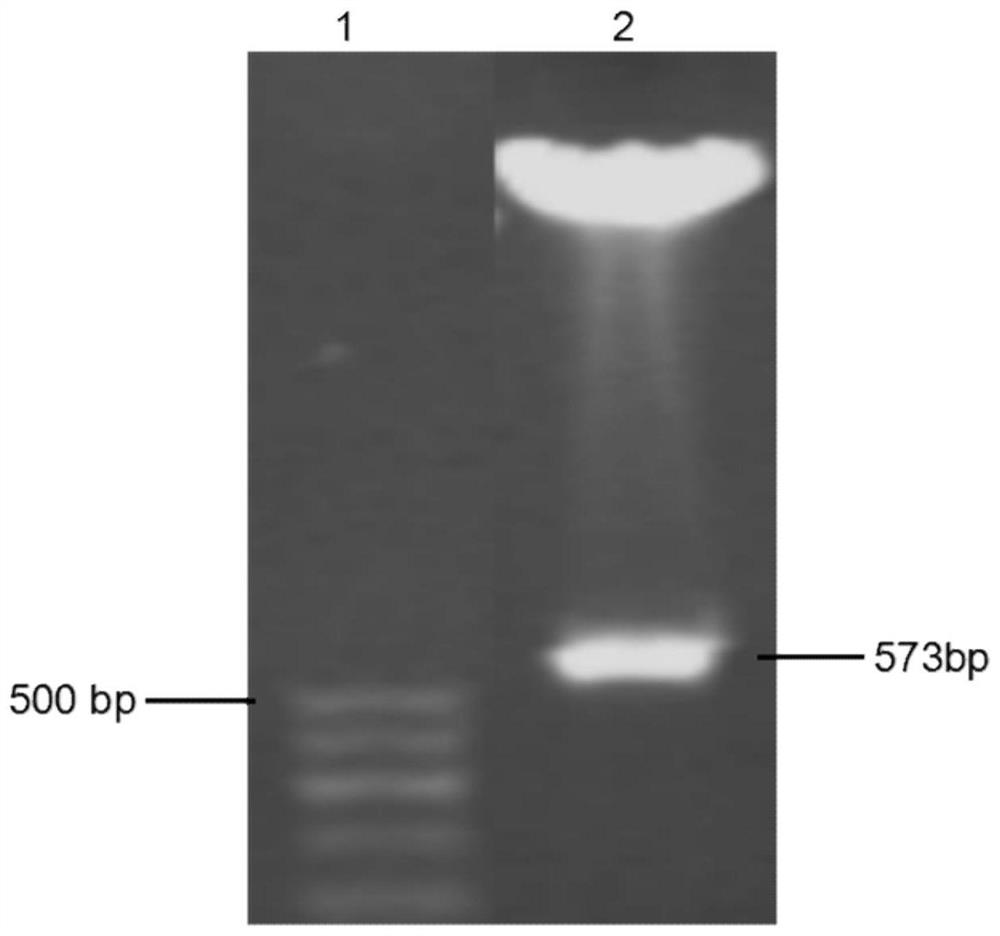
[0033] Example 1 Construction of Human Wnt5a-NL Nucleic Acid Recombinant
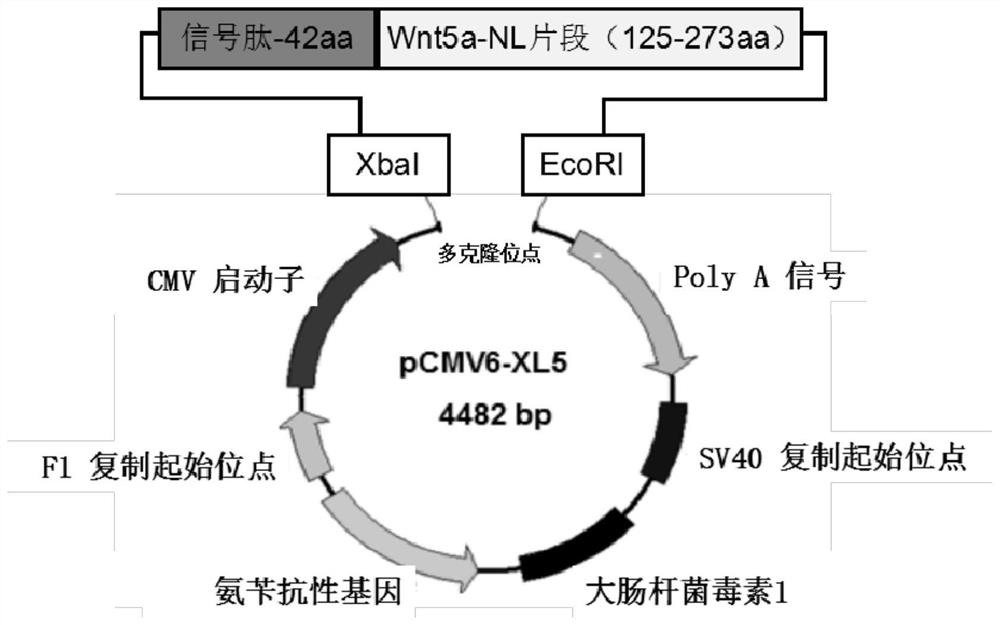
[0034] According to the characteristics of Wnt family protein structure, construct phWnt5a-NL nucleic acid recombinant, such as figure 1 As shown, the selected target expression vector is pCMV6-XL5, and the target fragment used is a fragment of human Wnt5a N-terminal linker (NTD-Linker, NL) (125-273aa, SEQ ID NO: 1). First, the amino acid and cDNA full-length sequences of hWnt5a were obtained from the BLAST database, and the amino acid sequence of hWnt5a was input into the SignalP online signal peptide prediction server to obtain the signal peptide sequence of hWnt5a (SEQ ID NO:5). At both ends of the signal peptide and the cDNA sequence of the selected hWnt5a-NL region, artificially design primers according to the conventional primer design principles; add XbaI and EcoRI restriction internals to the 5' end of the upstream primer of the signal peptide and the downstream primer of the hWnt5a-NL fragment,...
Embodiment 2
[0040] Embodiment 2 Amplification and extraction of human Wnt5a-NL nucleic acid recombinant in Escherichia coli
[0041] The Escherichia coli transformed with the recombinant plasmid was inoculated in 10 mL of LB medium (containing 100 mg / L of Amp + antibiotics), cultured overnight at 37°C with shaking at 250rpm. The next day, 10 mL of the overnight culture was transferred to 1 L of LB medium (containing 100 mg / L of Amp + Antibiotics), 37°C, 250rpm culture to OD 600 Value around 2.0. Bacteria were collected by centrifugation. The recombinant plasmid was extracted according to the instruction manual of plasmid extraction. The concentration and purity of the DNA were measured by an ultraviolet spectrophotometer to ensure that the ratio of the extracted plasmid A260 / A280 was between 1.8-2.0.
Embodiment 3
[0042] Example 3 In vivo and in vitro expression identification of human Wnt5a-NL nucleic acid recombinant
[0043] The recombinant plasmid prepared in Example 2 was transiently transfected into human 293T cells, and the cells were collected 48 hours later; the total protein was extracted according to the instructions of the protein extraction kit; the expression product was identified in vitro by Western blot. Ensure that the confluence of the cells is 70%-90% during transfection; dilute 1 μg of plasmid and 3 μL of Lipofectamine 2000 transfection reagent (purchased from Invitrogen, catalog number: 11668-027) with 250 mL of Opti-MEM respectively, and then mix them and incubate at room temperature 20min. After taking out the cells, discard the supernatant, wash with PBS once, add the mixed plasmid and transfection reagent mixture, and drop gently into the culture dish. After 4-5 hours, add DMEM complete medium to culture overnight, replace the medium once with complete medium,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com