Reduction-response self-depolymerization graft polymer based on polysaccharide as well as preparation method and application thereof
A graft polymer and polymer technology, applied in the field of polysaccharide-based reduction-response self-depolymerization graft polymer and its preparation, can solve the problems of macromolecular hydrophobic polymers that are not easy to metabolize, long-term toxicity, and cumbersome synthesis steps , to achieve the effect of high efficiency, non-toxicity and promotion of drug release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
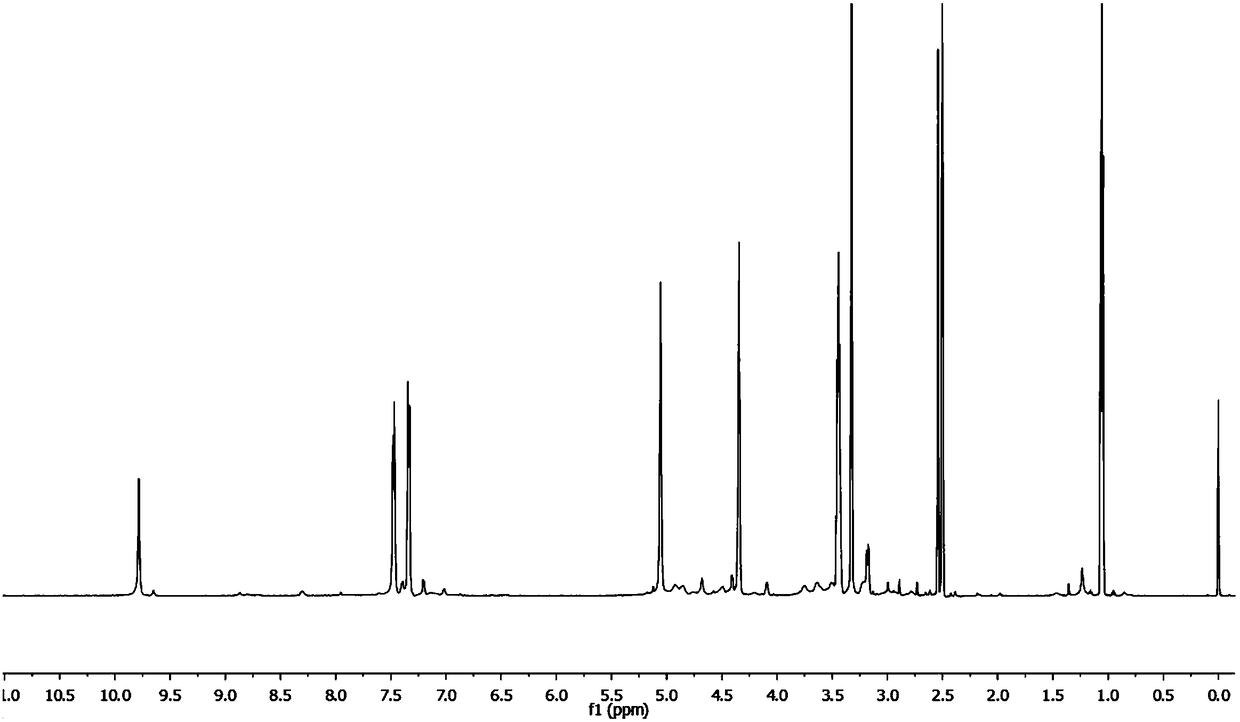
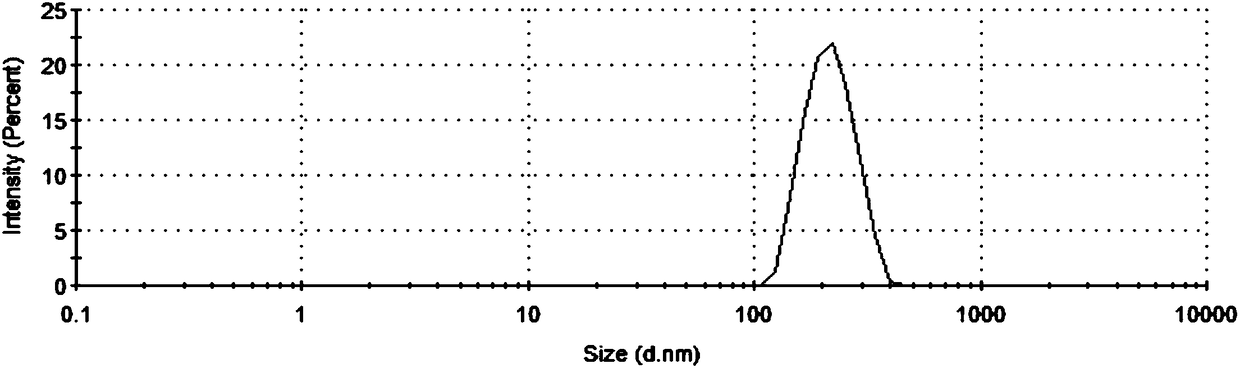
Image
Examples
preparation example Construction
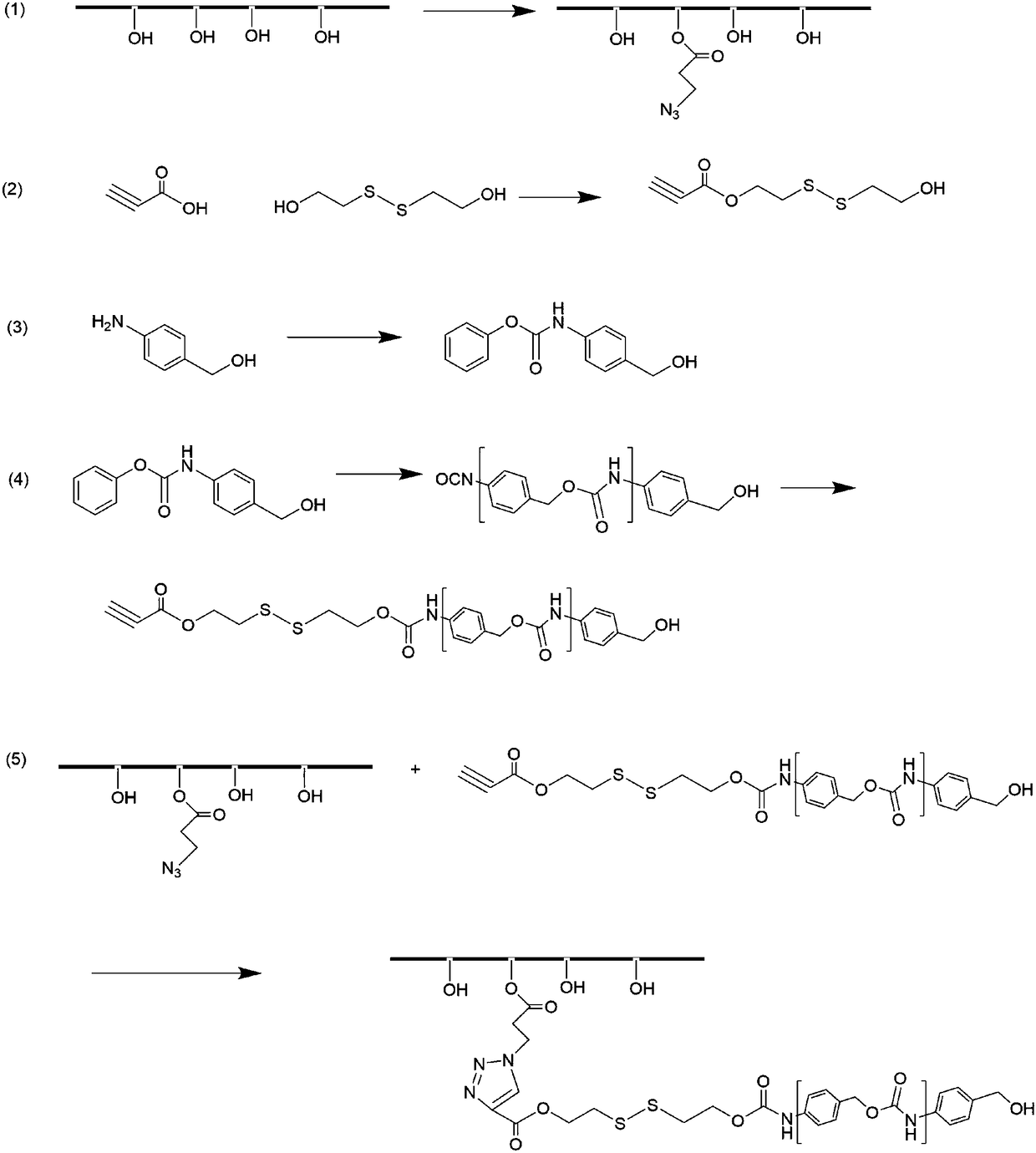
[0041] A method for preparing polysaccharide-based reduction-responsive self-depolymerization graft polymers, the synthetic route is detailed in figure 1 , including the following steps:
[0042] 1) Dissolve polysaccharide and 3-azidopropionic acid in deionized water, add 1-ethyl-(3-dimethylaminopropyl) carbodiimide and N-hydroxysuccinimide, at 40 The reaction was carried out at -60°C for 24-72 hours, then dialyzed and freeze-dried to obtain polysaccharide-azide derivatives.
[0043] Wherein, the solid-to-liquid ratio of the polysaccharide, 3-azidopropionic acid, deionized water, 1-ethyl-(3-dimethylaminopropyl) carbodiimide and N-hydroxysuccinimide 1g: 0.1-0.5g: 20mL: 0.5g: 0.3g.
[0044] 2) dissolving propiolic acid and bis(2-hydroxyethyl) disulfide in tetrahydrofuran, then adding dicyclohexylcarbodiimide and 4-dimethylaminopyridine, reacting overnight at room temperature, filtering to remove the white precipitate, and using Silica gel column purification to obtain alkyne-...
Embodiment 1
[0056] (1) Dissolve 1g dextran, 0.3g 3-azidopropionic acid and 0.3g N-hydroxysuccinimide in 20mL deionized water, then add 0.5g 1-ethyl-(3-dimethyl Aminopropyl) carbodiimide was reacted at 45°C for 48 hours, dialyzed with a dialysis bag for three days, and freeze-dried to obtain dextran-azide derivatives.
[0057] (2) Dissolve 2g of propiolic acid and 10g of bis(2-hydroxyethyl)disulfide in 20mL of tetrahydrofuran, then add 7.6g of dicyclohexylcarbodiimide and 0.4g of 4-dimethylaminopyridine, and react overnight at room temperature , filtered to remove the white precipitate, rotary evaporated to remove tetrahydrofuran, and then purified with a silica gel column (mobile phase was ethyl acetate and n-hexane) to obtain the product alkyne-disulfide bond-hydroxyl.
[0058] (3) Dissolve 1g of 4-aminobenzyl alcohol in a mixed solution of 15mL tetrahydrofuran / saturated aqueous sodium bicarbonate solution, then add 1.2mL phenyl chloroformate, react for 1h, add 50mL ethyl acetate, wash w...
Embodiment 2
[0063] (1) Dissolve 1 g of sodium alginate, 0.5 g of 3-azidopropionic acid and 0.3 g of N-hydroxysuccinimide in 20 mL of deionized water, then add 0.5 g of 1-ethyl-(3-dimethyl Aminopropyl) carbodiimide was reacted at 60°C for 24 hours, dialyzed with a dialysis bag for three days, and freeze-dried to obtain sodium alginate-azide derivatives.
[0064] (2) Dissolve 2g of propiolic acid and 10g of bis(2-hydroxyethyl)disulfide in 20mL of tetrahydrofuran, then add 7.6g of dicyclohexylcarbodiimide and 0.4g of 4-dimethylaminopyridine, and react overnight at room temperature , filtered to remove the white precipitate, rotary evaporated to remove tetrahydrofuran, and then purified with a silica gel column (mobile phase was ethyl acetate and n-hexane) to obtain the product alkyne-disulfide bond-hydroxyl.
[0065] (3) Dissolve 1g of 4-aminobenzyl alcohol in a mixed solution of 15mL tetrahydrofuran / saturated aqueous sodium bicarbonate solution, then add 1.2mL phenyl chloroformate, react fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com