Biosynthesis preparation method of L-glufosinate
A biosynthesis and bio-enzyme technology, applied in the direction of fermentation, can solve the problems of high risk, soil compaction, and demanding processing and manufacturing processes, and achieve the effects of high substrate utilization, simple process flow, and good catalytic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 1. Preparation of nitrile hydratase
[0037]Recombinant nitrile hydratase genetically engineered bacteria, the specific preparation method is: select the gene sequence of nitrile hydratase derived from Rhodococcus. Synthetic), cloned into the Nde I and XhoI restriction sites of the expression vector pET28a, and transformed the host strain E.coli BL21 (DE3) competent cells; after picking the positive transformant and identifying it by sequencing, the recombinant expression vector was obtained; the recombinant expression The vector is transferred into E. coli BL21 (DE3) strain, and the recombinant nitrile hydratase gene engineering bacteria capable of inducing the expression of the recombinant nitrile hydratase is obtained.
[0038] Inoculate the recombinant nitrile hydratase genetically engineered bacteria into LB medium containing kanamycin, and culture overnight at 37°C to obtain seed culture solution; inoculate the seed culture solution into TB medium containing kanam...
Embodiment 2
[0052] The biosynthetic preparation method of L-glufosinate-ammonium comprises the steps:
[0053] Step 1: The reaction was carried out in a 1L shake flask, with 30g of 2-amino-4-(ethoxymethylphosphono)-butyronitrile as the substrate, and 300mL of citric acid-sodium citrate buffer solution as the solvent for resuspension 3g whole cells of nitrile hydratase genetic engineering bacteria derived from Rhodococcus.rhodochrous, 3g whole cells of amide racemase genetic engineering bacteria derived from Achromobacter obae, 3g whole cells of L-amide hydrolase genetic engineering bacteria derived from Brevundimonas diminuta Cells, put pyridoxal phosphate into the shake flask, the input concentration of pyridoxal phosphate is 10mM, then add CoCl 2 , CoCl 2 The input concentration is 1mM, the pH value of the transformation system is controlled to be 6.5, and the temperature of the transformation system is controlled to be 35°C; the transformation reaction is carried out in a shaking tabl...
Embodiment 3
[0058] The biosynthetic preparation method of L-glufosinate-ammonium comprises the steps:
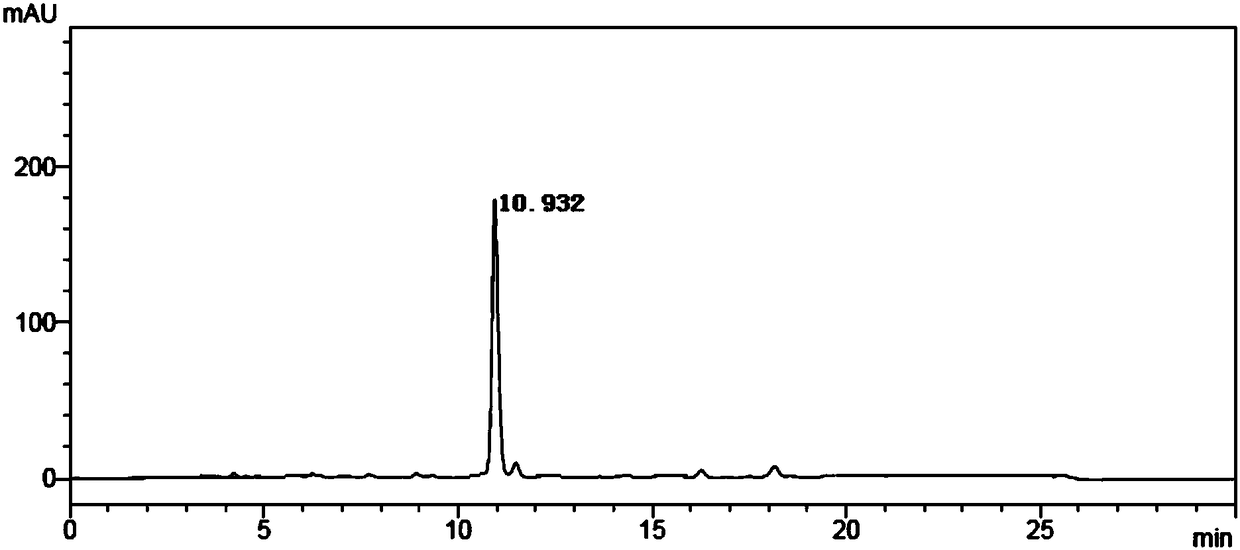
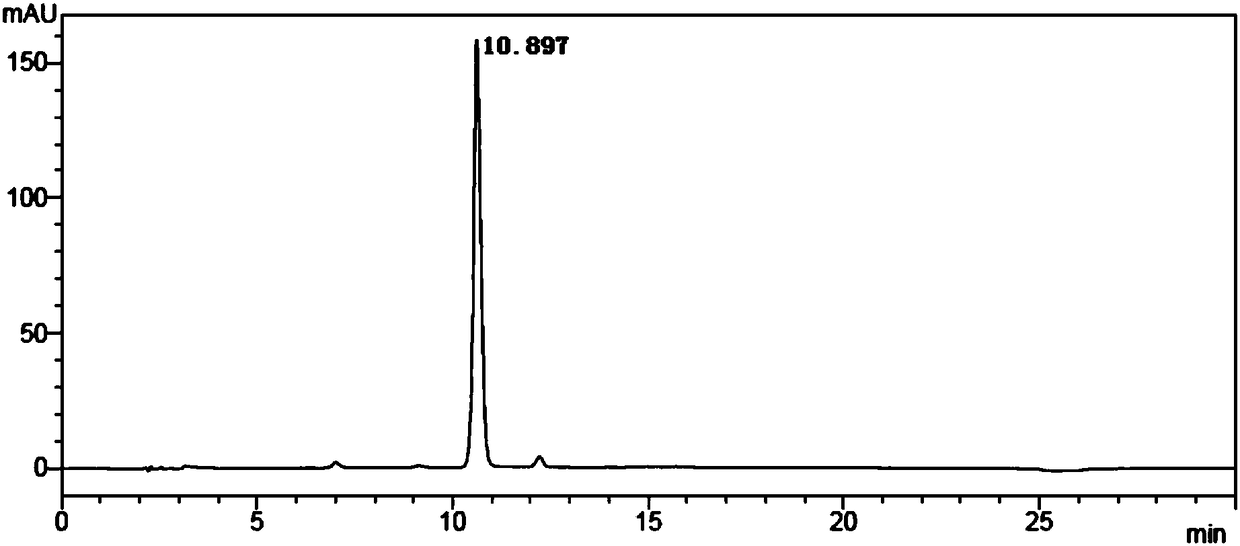
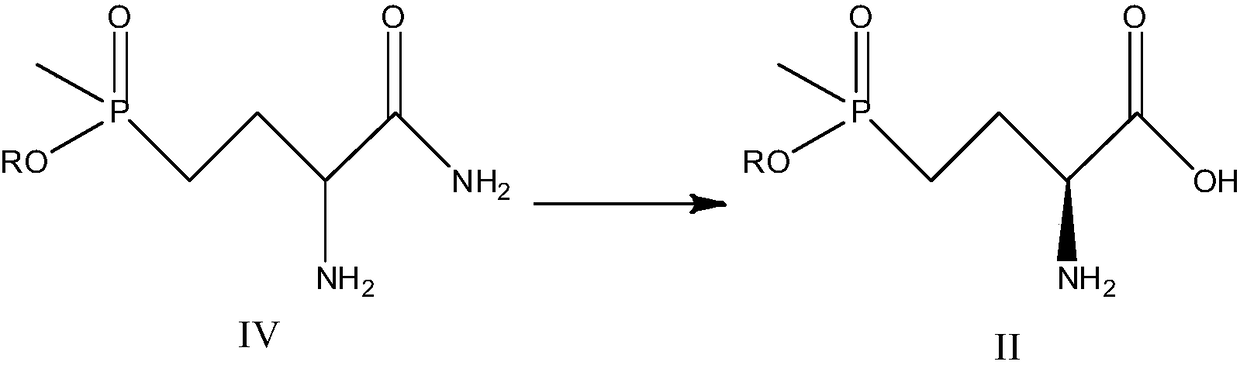
[0059] Step 1: The reaction was carried out in a 500mL shake flask, with 20g of 2-amino-4-(ethoxymethylphosphono)-butyronitrile as a substrate, and 200mL of phosphate buffer solution resuspended 2g of Rhodococcus. The whole cell of rhodochrous nitrile hydratase genetically engineered bacteria, the pH value of the transformation system is controlled to be 8, and the temperature of the transformation system is controlled to be 40°C; the transformation reaction is carried out in a shaking table, and the speed of the shaking table is controlled at 180r / min. The product was completely consumed, and purified to obtain 2-amino-4-(ethoxymethylphosphono)-butyramide. MS(ESI): m / z 209.11[M+H] + The reaction formula is as follows:
[0060]
[0061] Step 2: The reaction was carried out in a 500 mL shake flask, with 10 g of purified 2-amino-4-(ethoxymethylphosphono)-butyramide as the substrate, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com