Local anesthesia pain relieving and slow-release medicine delivery system as well as preparation method and application thereof
A local anesthesia and drug delivery system technology, applied in the field of medicine, can solve the problems of osmotic pressure reduction, interference, etc., and achieve the effects of uniform particle size, good sustained release effect, high encapsulation efficiency and drug loading capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
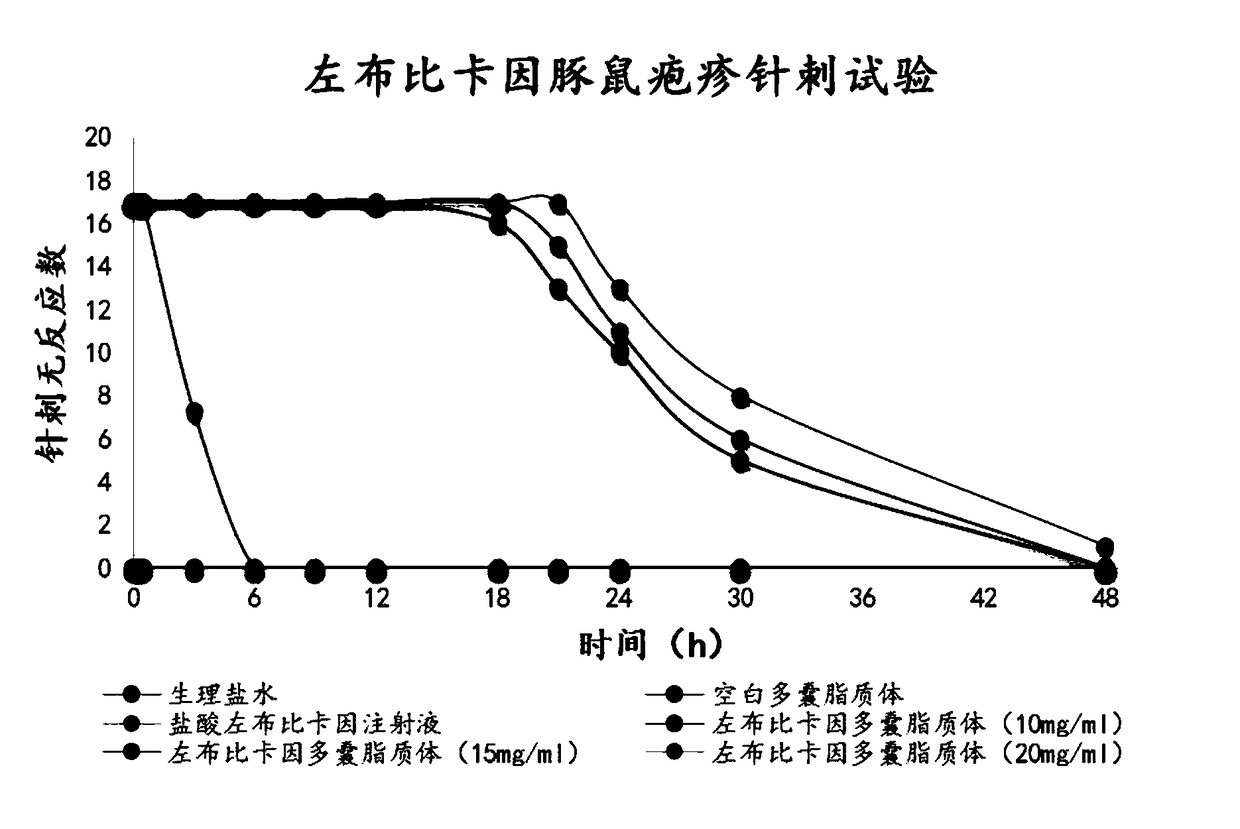
Image
Examples
Embodiment 1
[0038] Prescription composition:
[0039]
[0040]
[0041] The preparation method of multivesicular liposome is as follows:
[0042] (1) Preparation of the inner aqueous phase
[0043]Weigh a certain amount of levobupivacaine hydrochloride, dissolve it in water, add 1M sodium hydroxide, and when no more insoluble matter appears, filter and collect the solid, wash with water for injection until neutral, dry at 60°C, and add to the solid Add other substances in the inner water phase to the powder, add 3ml of 1M phosphoric acid, dissolve and add water to 5ml to obtain the water phase.
[0044] (2) Preparation of the external aqueous phase
[0045] Weigh niacin and sodium citrate in the outer aqueous phase in the above table, add 80ml of water to dissolve, adjust the pH to 7.4 with arginine, add water to 100ml, and obtain the outer aqueous phase.
[0046] (3) Preparation of oil phase
[0047] Weigh the substances in the oil phase in the above table, and dissolve them in...
Embodiment 2
[0053] Prescription composition:
[0054]
[0055] The preparation method of multivesicular liposome is as follows:
[0056] (1) Preparation of the inner aqueous phase
[0057] Weigh 200 mg of bupivacaine, add other substances in the inner water phase, add 2.5 ml of 1M nitric acid, dissolve and add water to 5 ml to obtain an aqueous phase.
[0058] (2) Preparation of the external aqueous phase
[0059] Weigh glucuronic acid, sucrose and sodium citrate from the outer aqueous phase in the above table, add 80ml of water to dissolve, adjust the pH to 8.0 with arginine, add water to 100ml, and obtain the outer aqueous phase.
[0060] (3) Preparation of oil phase
[0061] Weigh the substances in the oil phase in the above table, and dissolve them in a mixed solution of 5 ml of chloroform and ether (volume ratio 4:1) to obtain the oil phase.
[0062] (4) Preparation of colostrum
[0063] The prepared internal water phase was added to the oil phase, and sheared at 16000 rpm fo...
Embodiment 3
[0067] Prescription composition:
[0068]
[0069] The preparation method of multivesicular liposome is as follows:
[0070] (1) Preparation of the inner aqueous phase
[0071] Weigh 250 mg of ropivacaine, add other substances in the inner water phase, add 3.5 ml of 1M phosphoric acid, dissolve and add water to 5 ml to obtain the water phase.
[0072] (2) Preparation of the external aqueous phase
[0073] Weigh vitamin C, sucrose and sodium tartrate from the outer water phase in the above table, add 80ml of water to dissolve, adjust the pH to 8.5 with lysine, add water to 100ml, and obtain the outer water phase.
[0074] (3) Preparation of oil phase
[0075] Weigh each substance of the oil phase in the above table and dissolve it with 5ml of chloroform to obtain the oil phase.
[0076] (4) Preparation of colostrum
[0077] The prepared internal water phase was added to the oil phase, and sheared for 10 min at 13000 rpm to obtain colostrum.
[0078] (5) Preparation of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com