PCV2d (porcine circovirus type 2) virus-like particle vaccine and preparation method thereof
A porcine circovirus and virus-like technology, applied in the field of porcine circovirus 2d virus-like particle vaccine and its preparation, can solve the problems of immune evasion, the impact of disease prevention and control in pig farms, and the inability to effectively prevent and control the prevalence of PCV2d, etc. To achieve a good protective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] ——Expression of PCV2d Cap protein
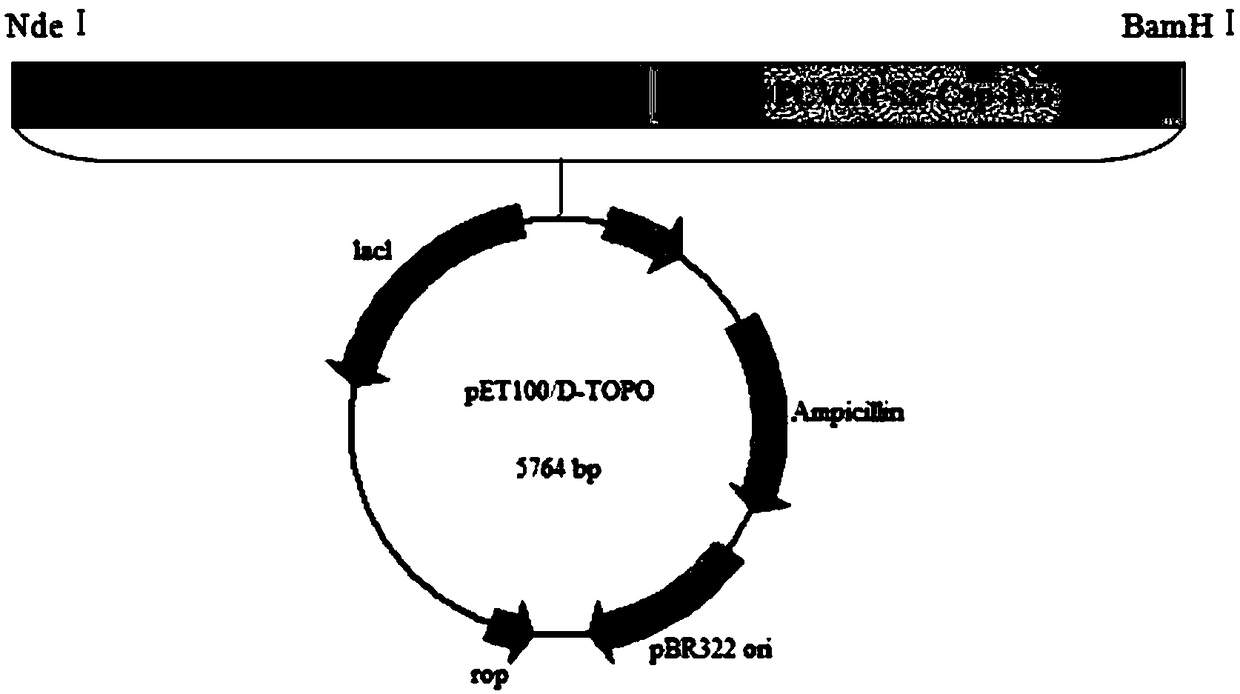
[0085] 1) Transform the pET100-PCV 2d-SS-Cap-Pro recombinant expression plasmid into BL21(DE3) Escherichia coli (Beijing Quanshijin Company) competent cells, and spread it on LB cultured with ampicillin at a concentration of 100 μg / mL basal plate, 37 ℃ inverted culture 12 ~ 14h.
[0086] 2) Randomly pick a monoclonal colony to 10 mL of LB medium containing 100 μg / mL ampicillin antibiotic, and shake and culture at 220 r / min at 37° C. for 12 hours.
[0087] 3) According to the ratio of (V / V) 1:100, add 10 mL of bacterial solution to 1 L of α-lactose fermentation medium (0.01 mol / L) containing 100 μg / mL ampicillin antibiotic that has been autoclaved, 37 ° C 180 r Centrifuge at 8,000g for 10 minutes at 4°C, discard the supernatant, and store the E. coli pellet at -80°C for use. protein purification.
Embodiment 2
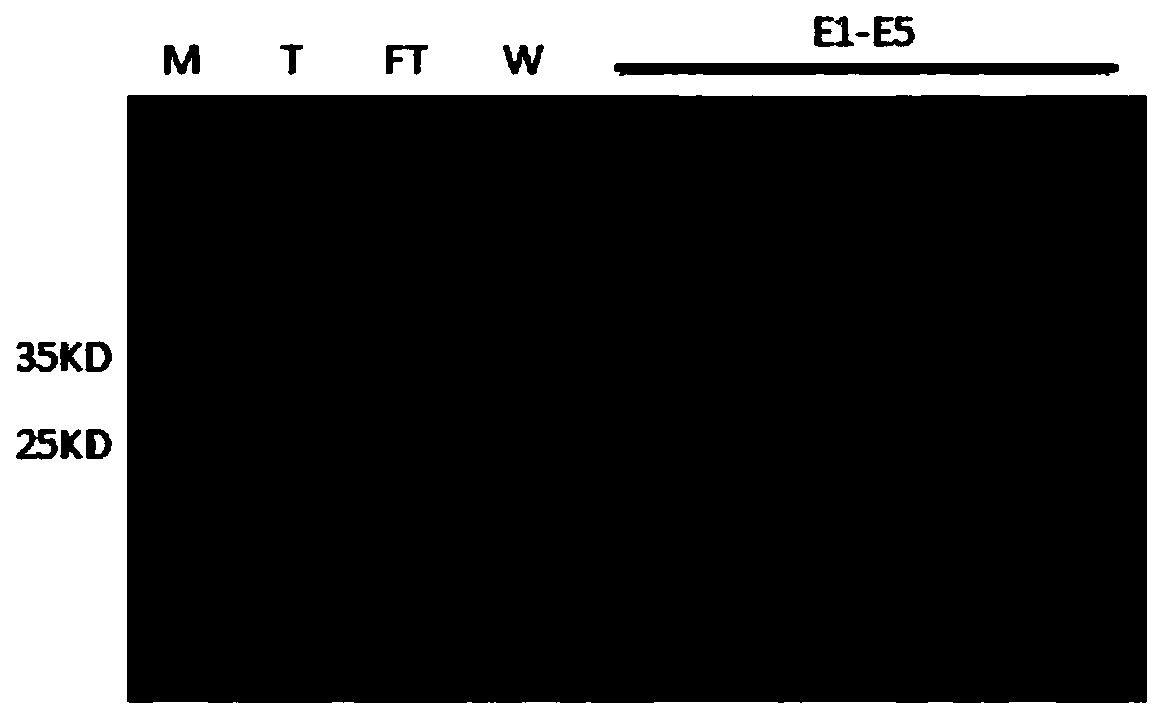
[0089] ——Purification of PCV 2d Cap protein by affinity chromatography
[0090] 1) The Escherichia coli precipitate is placed on an ice bath, and 100mL of PBS lysis buffer (Lysis buffer, its formula is: lysis buffer: NaCl 500mM, NaCl 2 HPO 4 100mM, Imidazole 30mM, KH 2 PO 4 100mM, Triton X-1001% (freshly added), pH 8.0) to lyse the centrifuged precipitate of 1L culture solution (concentrated according to the volume ratio of 1:10 times), and fully suspend the bacterial precipitate.
[0091] 2) The suspended sample is crushed and precipitated by a low-temperature ultra-high pressure continuous flow cell crusher, and the pressure is 1300bar, and the crushing is repeated 6 to 8 times. The crushed product is stained by Gram staining method, and more than 95% of E. coli are completely crushed under a microscope. The samples were then centrifuged at 16,000 g for 20 min at 4°C, and the supernatant was collected after centrifugation.
[0092] 3) Wash the Ni-NTA filler with 3 to 5...
Embodiment 3
[0101] - Assembly of PCV 2d virus-like particles
[0102] After performing SDS-PAGE analysis on the protein purified by affinity chromatography prepared in Example 2, select a high-concentration and high-purity protein and place it in a 7000D size dialysis bag and place it in a 4°C freezer in the assembly buffer of the present invention (Ammoniumcitrate 200mM, Na 3 PO 4 100mM, Tris 20mM, Arginine 20mM, pH7.0) in dialysis for 48h, the sample collected after dialysis was negatively stained with 1% phosphotungstic acid, and the virus-like particles were observed under a transmission electron microscope.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com